- All four lead pipeline programs progressing towards expected clinical milestones in 2019
- Gearing up for top-line data from the pivotal Phase 3 study of Sci-B-Vac®, VBI’s trivalent hepatitis B vaccine, expected mid-year 2019
- Expanded Part A data from GBM Phase 1/2a study of VBI-1901 to be presented at ASCO
- Part B of GBM Phase 1/2a study expected to initiate mid-year 2019
- Hepatitis B immunotherapeutic remains on track for initiation of Phase 2 proof-of-concept study by year-end 2019
VBI Vaccines Inc. (NASDAQ: VBIV) (“VBI”), a commercial-stage biopharmaceutical company developing next-generation infectious disease and immuno-oncology vaccines, today reported financial results for the first quarter ending March 31, 2019, and highlighted progress of the company’s pipeline.
“2019 has the potential to be a transformative year for VBI, marked by clinical milestones across all four of our lead programs, and as such, the first quarter of 2019 was characterized by intense focus on the execution of our ongoing clinical programs,” said Jeff Baxter, President and CEO, VBI Vaccines Inc. “In April 2019, all subjects in the Sci-B-Vac® pivotal Phase 3 PROTECT study completed clinical visits, including follow-up visits for safety, which confirms the timeline to top-line data. This data read-out is the most significant clinical milestone in the history of VBI and we remain diligently focused and excited as we advance towards the data read-out, expected mid-year this year, 2019.”
Recent Highlights and Upcoming Milestones
Sci-B-Vac®: Trivalent Prophylactic Hepatitis B Vaccine
Sci-B-Vac® is currently being evaluated in a global, pivotal Phase 3 clinical program, the results of which are intended to support future regulatory and marketing authorization submissions in the U.S., Europe, and Canada. The program consists of two concurrent Phase 3 studies – PROTECT and CONSTANT.
PROTECT: 2-arm safety and immunogenicity study in approximately 1,500 adults age 18 and older
- Top-line data, expected mid-year 2019, will include both co-primary endpoints and key secondary endpoints.
- Co-primary endpoints: After three doses of Sci-B-Vac® vs. three doses of Engerix-B®, (i) non-inferiority of seroprotection rates in adults age 18 and older, and (ii) superiority of seroprotection rates in adults over the age of 45.
- Secondary endpoints: (i) non-inferiority of seroprotection rates after two doses of Sci-B-Vac® vs. three doses of Engerix-B®, and (ii) safety and tolerability.
- In April 2019, VBI presented data in a poster presentation at The International Liver Congress™ (ILC), the Annual Meeting of the European Association for the Study of the Liver (EASL), which support the secondary endpoints in PROTECT. The poster illustrated data from three previously-conducted clinical studies – two randomized Phase 3 studies comparing Sci-B-Vac® to Engerix-B® conducted in Vietnam and Russia, and one single-arm Phase 4 study conducted in Israel – in subjects aged 18 to 45 years. Data from all three studies demonstrated a clean safety profile for Sci-B-Vac® and seroprotection rates of more than 98% after two vaccinations in all subjects receiving Sci-B-Vac®.
CONSTANT: 4-arm lot-to-lot consistency study in approximately 2,850 subjects
- The primary endpoint is to demonstrate consistency of immune response, measured by geometric mean concentration (GMC), across three independent, consecutively manufactured lots of Sci-B-Vac®.
- Top-line CONSTANT data is expected around year-end 2019.
VBI-1901 – Glioblastoma (GBM) Immunotherapeutic
VBI-1901 is currently being evaluated in a two-part Phase 1/2a study in recurrent GBM patients.
PART A: Dose-escalation phase designed to evaluate the safety, tolerability, and to define the optimal therapeutic dose level of VBI-1901
- Expanded data from Part A of the study was recently selected for poster presentation at the Annual Meeting of the American Society of Clinical Oncology (ASCO) in early June, and will feature expanded immunologic data along with tumor and clinical responses, based on MRIs and survival data, from all three dose cohorts in Part A of the study.
- The poster, number 237, will be presented during the Central Nervous System Tumors session on Sunday, June 2, 2019 from 8:00 AM ET to 11:00 AM ET.
PART B: Subsequent extension phase with a narrower enrollment criteria, designed to more clearly assess immunologic responses and the correlation with tumor and clinical responses, based on MRIs and survival data
- As announced in April 2019, based on safety and immunogenicity data, the highest dose tested in Part A of the study, 10mcg, has been selected as the optimal dose level to test in Part B of the study.
- Enrollment in Part B will be restricted only to those with a first tumor recurrence.
- Initiation of enrollment of the 10 patients in Part B of the study is expected mid-year 2019.
VBI-2601 – Hepatitis B Immunotherapeutic
- In December 2018, VBI announced a license and collaboration agreement with Brii Biosciences for the development of a functional cure for chronic hepatitis B using VBI-2601, the company’s novel immunotherapeutic candidate formulated to target and enhance B- and T-cell immunity.
- In January 2019, VBI initiated pre-clinical studies required to enable initiation of a Phase 2 human proof-of-concept study, which is expected to initiate around year-end 2019.
VBI-1501 – Prophylactic Cytomegalovirus (CMV) Vaccine
- In December 2018, VBI announced plans for a Phase 2 dose-ranging study following positive discussions with Health Canada, and the company anticipates similar discussions with the FDA in 2019.
- The Phase 2 study is expected to assess the safety and immunogenicity of higher dosages of VBI-1501, up to 20mcg, with study initiation anticipated around the end of 2019.
- A toxicology study to support the new dose levels is underway, the results of which are required prior to the start of the Phase 2 study.
First Quarter 2019 Financial Results
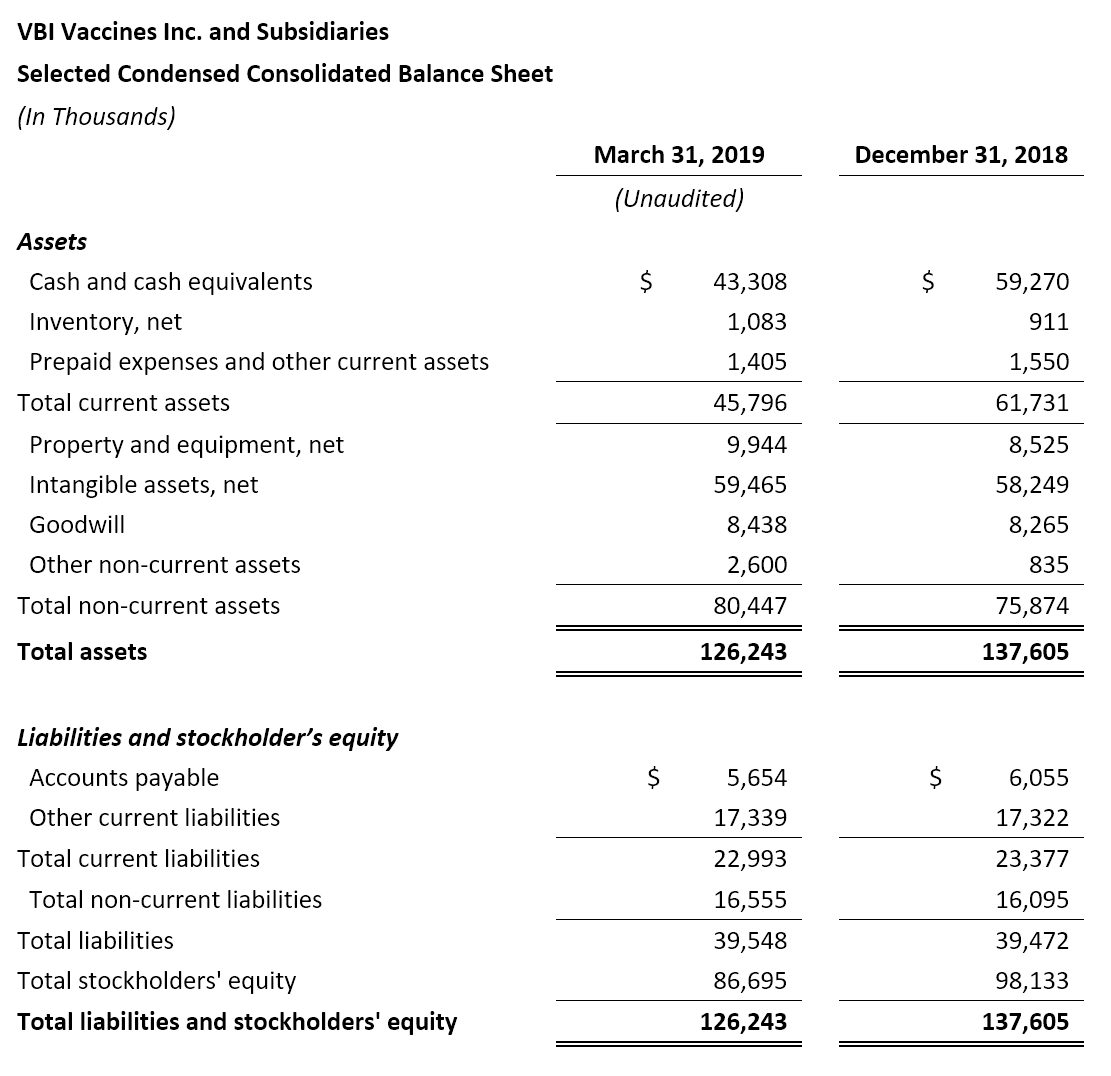
- Cash Position: VBI ended the first quarter of 2019 with $43.3 million in cash and cash equivalents compared to $59.3 million as of December 31, 2018.
- Net Cash Used in Operating Activities: Net cash used in operations for the three months ended March 31, 2019 was $14.0 million compared to $8.6 million for the same period in 2018.
- Cash Used for Purchase of Property and Equipment: Cash used for the purchase of property and equipment was $1.9 million for the three months ended March 31, 2019 compared to $1.0 million for the same period in 2018. The increase in spend is due to the modernization and capacity increase of the company’s manufacturing facility in Rehovot, Israel. The construction and temporary closure of the facility began in Q2 2018 and is now substantially complete. We anticipate being able to recommence operations in the facility by the end of 2019.
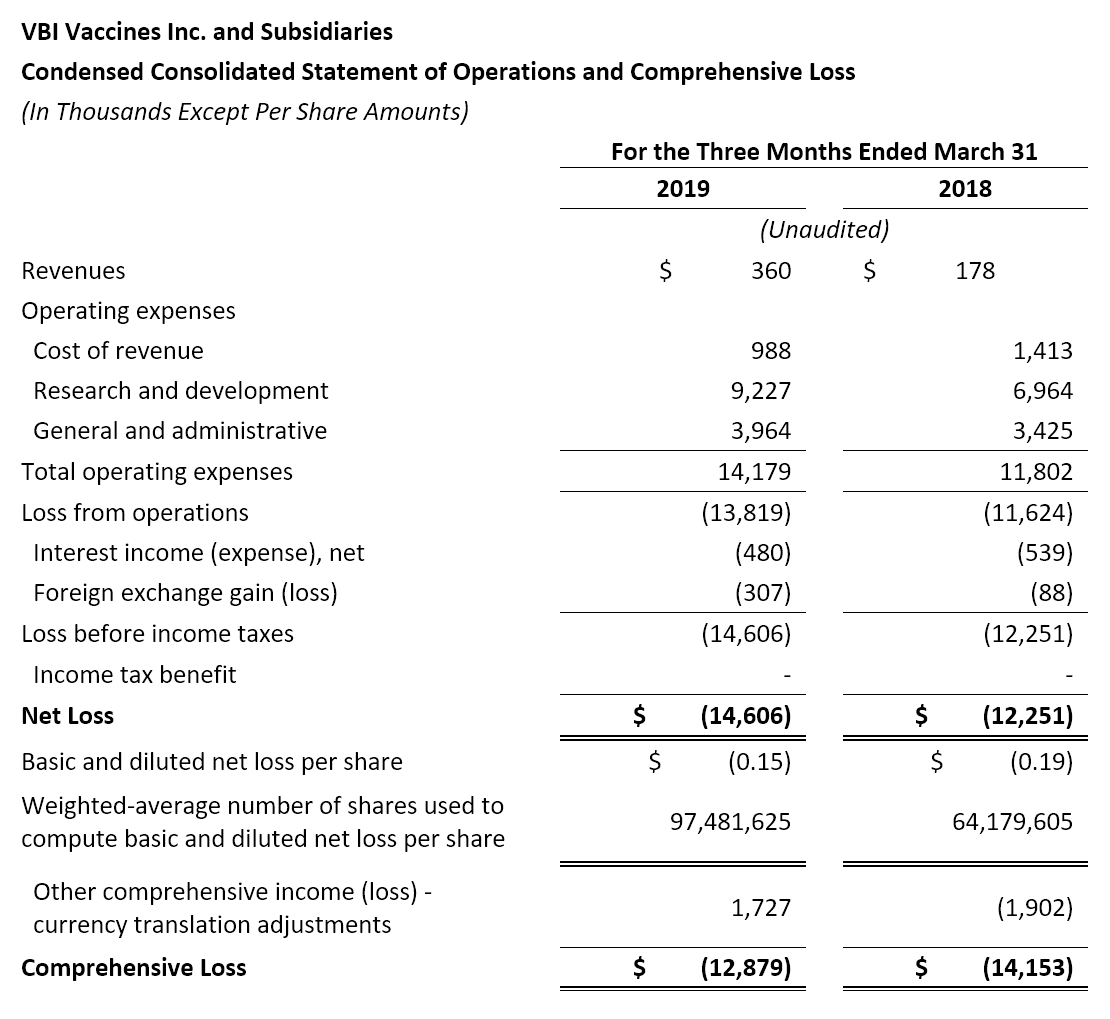
- Revenue: Revenue in the first quarter of 2019 was $0.4 million, compared to $0.2 million for the same period in 2018. The increase was primarily due to R&D service revenues earned pursuant to the therapeutic hepatitis B license and collaboration agreement with Brii Biosciences, offset by a slight decrease in named-patient sales of Sci-B-Vac® in Europe.
- Research and Development (R&D): R&D expenses were $9.2 million for the first quarter of 2019, compared to $7.0 million for the same period in 2018. The increase was primarily due to the advancement of the Phase 3 program for Sci-B-Vac® and the Phase 1/2a clinical study for VBI-1901 in recurrent GBM patients.
- General and Administrative (G&A): G&A expenses were $4.0 million for the first quarter of 2019, compared to $3.4 million for the same period in 2018. The increase was primarily due to increased human resource expenses, the allocation of certain cost of revenues related to the temporary Rehovot facility closure, and pre-commercialization costs for Sci-B-Vac®.
- Net Loss: Net loss and net loss per share for the first quarter of 2019 were $14.6 million and $0.15, respectively, compared to a net loss of $12.3 million and a net loss per share of $0.19 for the first quarter of 2018.
About VBI Vaccines Inc.
VBI Vaccines Inc. (Nasdaq: VBIV) is a commercial-stage biopharmaceutical company developing a next generation of vaccines to address unmet needs in infectious disease and immuno-oncology. VBI is advancing the prevention and treatment of hepatitis B, with the only commercially-approved trivalent hepatitis B vaccine, Sci-B-Vac®, which is approved for use in Israel and 10 other countries and is currently in a Phase 3 study in the U.S., Europe, and Canada, and with an immunotherapeutic in development for a functional cure for chronic hepatitis B. VBI’s eVLP Platform technology allows for the development of enveloped virus-like particle (eVLP) vaccines that closely mimic the target virus to elicit a potent immune response. Integrating its cytomegalovirus (CMV) expertise with the eVLP platform technology, VBI’s lead eVLP vaccine candidates include a prophylactic CMV vaccine candidate and a therapeutic glioblastoma (GBM) vaccine candidate. VBI is headquartered in Cambridge, MA with research operations in Ottawa, Canada and research and manufacturing facilities in Rehovot, Israel.
Cautionary Statement on Forward-looking Information
Certain statements in this press release that are forward-looking and not statements of historical fact are forward-looking statements within the meaning of the safe harbor provisions of the Private Securities Litigation Reform Act of 1995 and are forward-looking information within the meaning of Canadian securities laws (collectively, “forward-looking statements”). The company cautions that such statements involve risks and uncertainties that may materially affect the company’s results of operations. Such forward-looking statements are based on the beliefs of management as well as assumptions made by and information currently available to management. Actual results could differ materially from those contemplated by the forward-looking statements as a result of certain factors, including but not limited to the ability to establish that potential products are efficacious or safe in preclinical or clinical trials; the ability to establish or maintain collaborations on the development of therapeutic candidates; the ability to obtain appropriate or necessary governmental approvals to market potential products; the ability to obtain future funding for developmental products and working capital and to obtain such funding on commercially reasonable terms; the company’s ability to manufacture product candidates on a commercial scale or in collaborations with third parties; changes in the size and nature of competitors; the ability to retain key executives and scientists; and the ability to secure and enforce legal rights related to the company’s products. A discussion of these and other factors, including risks and uncertainties with respect to the company, is set forth in the Company’s filings with the Securities and Exchange Commission and the Canadian securities authorities, including its Annual Report on Form 10-K filed with the Securities and Exchange Commission on February 25, 2019, and filed with the Canadian security authorities at sedar.com on February 25, 2019, as may be supplemented or amended by the Company’s Quarterly Reports on Form 10-Q. Given these risks, uncertainties and factors, you are cautioned not to place undue reliance on such forward-looking statements, which are qualified in their entirety by this cautionary statement. All such forward-looking statements made herein are based on our current expectations and we undertake no duty or obligation to update or revise any forward-looking statements for any reason, except as required by law.
VBI Contact
Nicole Anderson, Communications Executive
Phone: (617) 830-3031 x124
Email: info@vbivaccines.com
VBI Investor Contact
Nell Beattie
Chief Business Officer
Email: IR@vbivaccines.com
VBI Media Contact
Burns McClellan, Inc.
Robert Flamm, Ph.D.
Phone: (212) 213-0006
Email: rflamm@burnsmc.com









