- Positive top-line data from PROTECT Phase 3 for Sci-B-Vac®, trivalent hepatitis B vaccine, announced June 2019 – top-line data from second Phase 3 study, CONSTANT, expected around year-end 2019
- ASCO presentation illustrated encouraging data from Part A of the ongoing Phase 1/2a study of VBI-1901 in recurrent glioblastoma (GBM) patients – first patient in Part B of the study was dosed in July 2019
- Therapeutic hepatitis B program remains on track for expected initiation of enrollment in a Phase 2 study in chronic hepatitis B patients in Q4 2019
VBI Vaccines Inc. (NASDAQ: VBIV) (“VBI”), a commercial-stage biopharmaceutical company developing next-generation infectious disease and immuno-oncology vaccines, today reported financial results for the second quarter ending June 30, 2019, and provided an update on the Company’s recent and future developments.
“In Q2 2019, we presented positive data on two of our lead programs – the successful PROTECT Phase 3 data for Sci-B-Vac®, our trivalent hepatitis B vaccine, and a poster presentation at ASCO illustrating encouraging data from Part A of the ongoing Phase 1/2a clinical study of VBI-1901 in recurrent GBM patients,” said Jeff Baxter, VBI’s president and CEO. “We continue to execute our corporate objectives and, based on the recent achievements and announcements, we believe the potential of the VBI pipeline is as strong as ever. We remain excited about VBI’s future, especially in light of the expected near-term milestones across all four lead programs.”
Recent Highlights and Upcoming Milestones
Sci-B-Vac®: Trivalent Prophylactic Hepatitis B Vaccine
Positive top-line data from PROTECT were announced in June 2019. The study, which enrolled a total of 1,607 adults, of which approximately 80% were age ≥ 45 years, successfully met both of its co-primary endpoints:
- Non-inferiority of seroprotection rates (SPR) of Sci-B-Vac® compared with Engerix-B® in all subjects age ≥ 18 years
- Superiority of SPR of Sci-B-Vac® compared with Engerix-B® in subjects age ≥ 45 years
Moreover, the SPR of Sci-B-Vac® compared with Engerix-B® was statistically significantly higher at each time point on a per-visit basis, and in all key subgroup analyses of adults age ≥ 18 years, including by age, gender, body mass index (BMI), diabetic status, and smoking status.
“The positive results from PROTECT reaffirm the robust safety and efficacy data that we have for Sci-B-Vac® from previous clinical studies, and they further support the meaningful commercial opportunity for the vaccine,” said Jeff Baxter. “We believe that, if approved, Sci-B-Vac® will be highly-competitive and well-positioned within the North American and European hepatitis B markets, differentiating on efficacy, safety, and cost. Where faster seroprotection and cost may drive use in the young and otherwise healthy populations, superior seroprotection rates and safety are likely to drive use in the older and immunocompromised populations. Pending successful completion of the CONSTANT Phase 3 study, we anticipate beginning the submissions of applications for regulatory approvals in the U.S., Europe, and Canada mid-year 2020.”
Top-line data from the CONSTANT Phase 3 study, a 4-arm lot-to-lot consistency study in approximately 2,850 subjects, is expected around year-end 2019.
VBI-1901 – Glioblastoma (GBM) Immunotherapeutic
Immunogenicity data and tumor/clinical responses from Part A of the ongoing Phase 1/2a study in recurrent GBM patients were presented at ASCO in June 2019. This data showed three out of six (3/6) patients in the high-dose, 10 μg, cohort had evidence of stable disease by magnetic resonance imaging (MRI) and median progression-free survival (PFS) was longer among vaccine responders (14.5 weeks) compared with non-responders (6 weeks).
Based on safety and immunogenicity, the highest dose tested in Part A, 10 µg, has been selected as the optimal dose level to test in Part B of the study. Part B of the study is a subsequent extension phase with narrower enrollment criteria, designed to more clearly assess immunologic responses and potential correlations with early clinical benefit. Enrollment of 10 first-recurrence GBM patients in Part B initiated in July 2019.
VBI-2601 – Hepatitis B Immunotherapeutic
As part of the collaboration with Brii Biosciences, work is underway to prepare regulatory filings to enable the expected initiation of enrollment in the Phase 2 study in subjects with chronic hepatitis B in the fourth quarter of 2019, which would thus provide an expected initial human proof-of-concept readout in the second half of 2020.
VBI-1501 – Prophylactic Cytomegalovirus (CMV) Vaccine
In July 2019, VBI received positive feedback from the FDA on the design of the proposed Phase 2 dose-ranging study that is expected to assess the safety and immunogenicity of dosages of VBI-1501 up to 20 µg with alum. Submission of the Investigational New Drug (IND) application for this study is expected around the end of 2019.
Enveloped Virus-Like Particle (eVLP) Platform
On August 8, 2019, at the Immuno-Oncology Summit 2019 in Boston, David E. Anderson, Ph.D., VBI’s Chief Scientific Officer, presented further data on the broad potential of the eVLP platform in immuno-oncology. The clinical and pre-clinical data presented showed that not only are eVLPs a highly potent antigen delivery mechanism, but the lipid bilayer surrounding the eVLPs also enables the functional activity of immunomodulatory molecules, such as CD40 ligand, which further enhances potency by activating both innate and adaptive immunity.
Second Quarter 2019 Financial Results
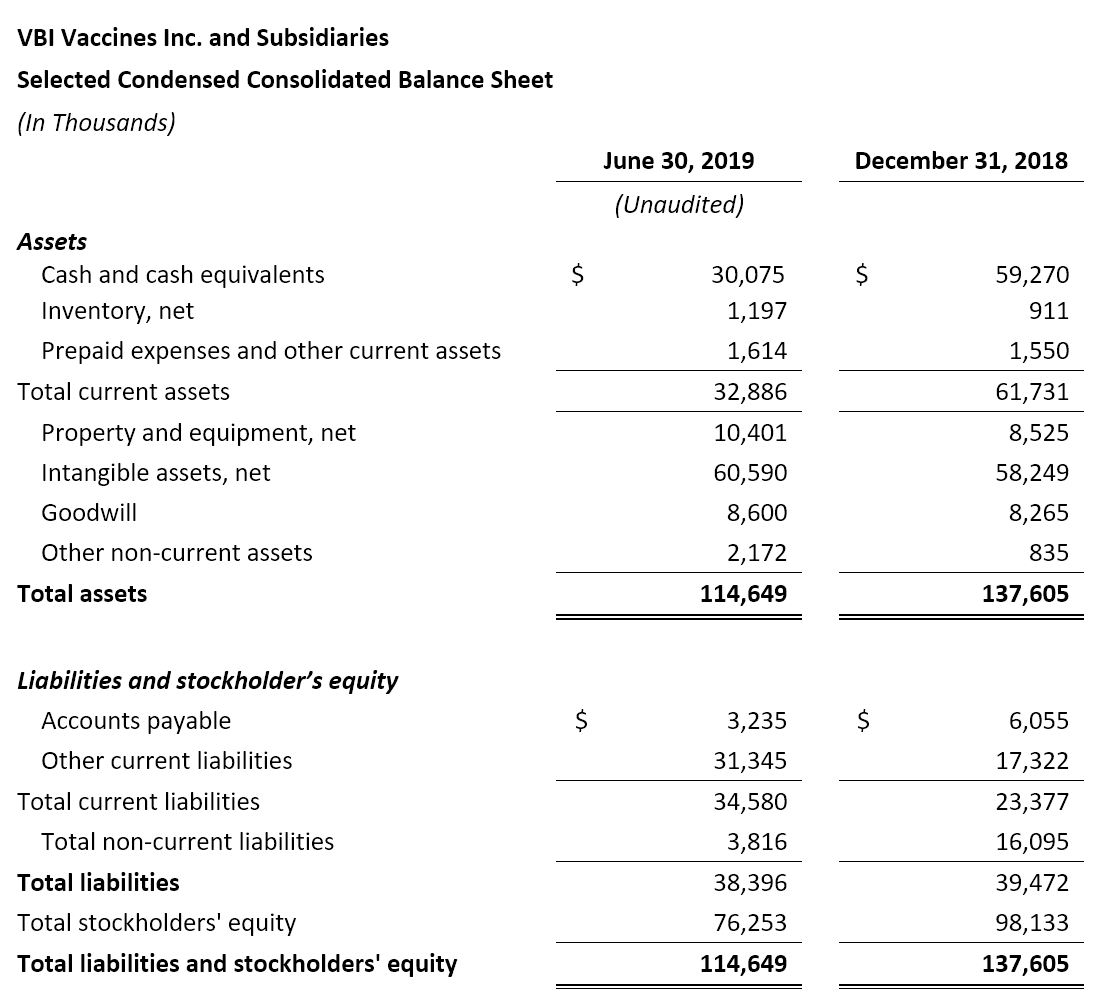
- Cash Position: VBI ended the second quarter of 2019 with $30.1 million in cash and cash equivalents compared to $59.3 million as of December 31, 2018.
- Net Cash Used in Operating Activities: Net cash used in operations for the six months ended June 30, 2019 was $26.3 million compared to $24.5 million for the same period in 2018.
- Cash Used for Purchase of Property and Equipment: Cash used for the purchase of property and equipment was $2.9 million for the six months ended June 30, 2019 compared to $2.0 million for the same period in 2018. The increase is largely related to the purchase of manufacturing and information technology equipment at the manufacturing facility in Rehovot, Israel, as part of the modernization and capacity increase of the facility. In 2018, the manufacturing facility in Rehovot was temporarily closed for modernization and capacity increase – operations re-commenced in May 2019, and we anticipate a review of the facility by the Israeli Ministry of Health in the second half of 2019.
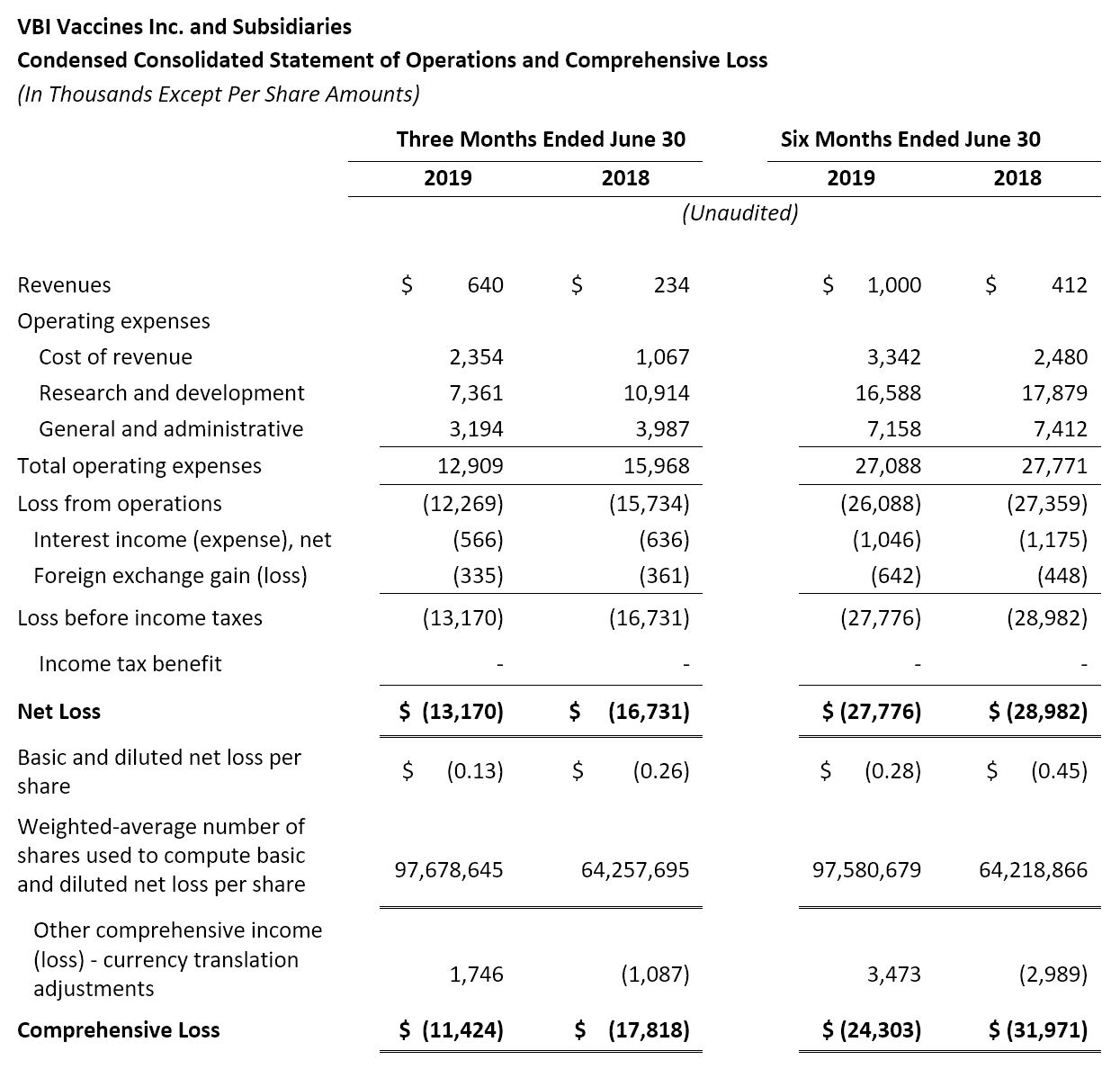
- Revenue: Revenue in the second quarter of 2019 was $0.6 million, compared to $0.2 million for the same period in 2018. The increase was primarily due to R&D services revenues earned pursuant to the therapeutic hepatitis B collaboration and license agreement with Brii Biosciences.
- Research and Development (R&D): R&D expenses were $7.4 million for the second quarter of 2019, compared to $10.9 million for the same period in 2018. The decrease was primarily due to the completion of the Phase 1 study assessing VBI-1501, and to the decrease in spending related to the advancement of the Phase 3 program for Sci-B-Vac® and the Phase 1/2a clinical study for VBI-1901.
- General and Administrative (G&A): G&A expenses were $3.2 million for the second quarter of 2019, compared to $4.0 million for the same period in 2018. The decrease was primarily due to a decrease in marketing and administrative expenses, as well as both an impairment loss on property and equipment and a reclassification of certain costs of revenues to G&A, as part of the temporary closure of the Rehovot facility, that were incurred in the three months ended June 30, 2018 that did not re-occur during the three months ended June 30, 2019.
- Net Loss: Net loss and net loss per share for the second quarter of 2019 were $13.2 million and $0.13, respectively, compared to a net loss of $16.7 million and a net loss per share of $0.26 for the second quarter of 2018.
About VBI Vaccines Inc.
VBI Vaccines Inc. (Nasdaq: VBIV) is a commercial-stage biopharmaceutical company developing a next generation of vaccines to address unmet needs in infectious disease and immuno-oncology. VBI is advancing the prevention and treatment of hepatitis B, with the only commercially-approved trivalent hepatitis B vaccine, Sci-B-Vac®, which is approved for use in Israel and 10 other countries and is currently in a Phase 3 program in the U.S., Europe, and Canada, and with an immunotherapeutic in development for a functional cure for chronic hepatitis B. VBI’s eVLP Platform technology allows for the development of enveloped virus-like particles (eVLP) that closely mimic the target virus to elicit a potent immune response. Integrating its cytomegalovirus (CMV) expertise with the eVLP platform technology, VBI’s lead eVLP program candidates include a prophylactic CMV vaccine candidate and a glioblastoma (GBM) vaccine immunotherapeutic candidate. VBI is headquartered in Cambridge, MA with research operations in Ottawa, Canada and research and manufacturing facilities in Rehovot, Israel.
Cautionary Statement on Forward-looking Information
Certain statements in this press release that are forward-looking and not statements of historical fact are forward-looking statements within the meaning of the safe harbor provisions of the Private Securities Litigation Reform Act of 1995 and are forward-looking information within the meaning of Canadian securities laws (collectively, “forward-looking statements”). The company cautions that such statements involve risks and uncertainties that may materially affect the company’s results of operations. Such forward-looking statements are based on the beliefs of management as well as assumptions made by and information currently available to management. Actual results could differ materially from those contemplated by the forward-looking statements as a result of certain factors, including but not limited to the ability to establish that potential products are efficacious or safe in preclinical or clinical trials; the ability to establish or maintain collaborations on the development of therapeutic candidates; the ability to obtain appropriate or necessary governmental approvals to market potential products; the ability to obtain future funding for developmental products and working capital and to obtain such funding on commercially reasonable terms; the company’s ability to manufacture product candidates on a commercial scale or in collaborations with third parties; changes in the size and nature of competitors; the ability to retain key executives and scientists; and the ability to secure and enforce legal rights related to the company’s products. A discussion of these and other factors, including risks and uncertainties with respect to the company, is set forth in the Company’s filings with the Securities and Exchange Commission and the Canadian securities authorities, including its Annual Report on Form 10-K filed with the Securities and Exchange Commission on February 25, 2019, and filed with the Canadian security authorities at sedar.com on February 25, 2019, as may be supplemented or amended by the Company’s Quarterly Reports on Form 10-Q. Given these risks, uncertainties and factors, you are cautioned not to place undue reliance on such forward-looking statements, which are qualified in their entirety by this cautionary statement. All such forward-looking statements made herein are based on our current expectations and we undertake no duty or obligation to update or revise any forward-looking statements for any reason, except as required by law.
VBI Investor & Media Contact
Nicole Anderson
Director, Corporate Communications & IR
Phone: (617) 830-3031 x124
Email: info@vbivaccines.com









