VBI Vaccines Inc. (Nasdaq: VBIV) (VBI), a commercial-stage biopharmaceutical company developing next-generation infectious disease and immuno-oncology vaccines, today announced the presentation of its abstract featuring data from one of the pivotal Phase 3 studies (PROTECT) evaluating Sci-B-Vac®, the company’s 3-antigen prophylactic hepatitis B (HBV) vaccine, in an e-poster presentation at the American Association for the Study of Liver Diseases (AASLD) – The Liver Meeting®, which took place on November 13, 2020.
Poster #: 0742
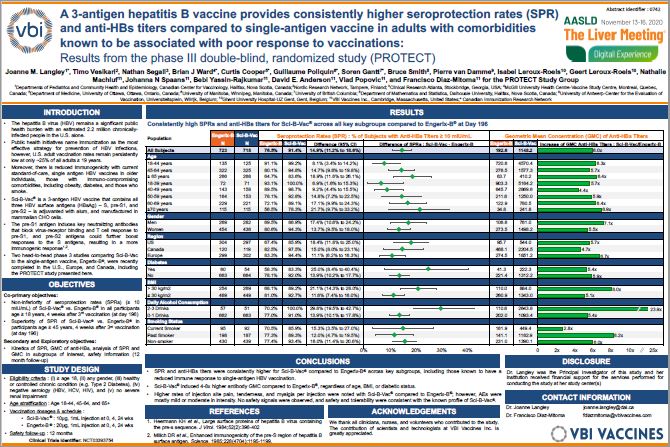
Title: A 3-antigen hepatitis B vaccine (3AV) provides consistently higher seroprotection rates (SPR) and anti-HBs titers compared to single-antigen vaccine in adults with comorbidities known to be associated with poor response to vaccinations: Results from the phase 3 double-blind, randomized study (PROTECT)
Joanne Langley, M.D., Professor of Pediatrics and Community Health and Epidemiology, CIHR-GSK Chair in Pediatric Vaccinology, Dalhousie University, and Head of the Division of Infectious Disease, IWK Health Centre, and principal investigator of the PROTECT study delivered the poster presentation.
“In the PROTECT study, the overall immunogenicity of Sci-B-Vac was higher than that of Engerix-B in all study participants; this is demonstrated across key subgroup populations where Sci-B-Vac induced consistently higher seroprotection rates and higher mean concentrations of hepatitis B surface antibodies,” said Dr. Langley. “These subgroup data are significant, as there is an unmet medical need in certain populations, including adults over the age of 45 and those with underlying comorbidities, who have known reduced immune responses to single-antigen hepatitis B vaccines.”
The poster highlighted Sci-B-Vac’s ability to induce higher peak (Day 196) seroprotection rates (SPRs) and serum levels of HBV surface antibodies (anti-HBs titers) compared to Engerix-B, persistence and durability of which are believed to be dependent upon peak levels induced. The data included:
- Consistently higher SPRs induced in all subjects vaccinated with Sci-B-Vac compared to Engerix-B:
- In all subjects, age 18+ : 91.4% vs. 76.5% (difference: 14.9%)
- Adults 18-44 years : 99.2% vs. 91.1% (difference: 8.1%)
- Adults 45-64 years : 94.8% vs. 80.1% (difference: 14.7%)
- Adults 65+ : 83.6% vs. 64.7% (difference: 18.9%)
- These results were consistent across notable subgroups, including diabetics (83.3% vs. 58.3%) and obese individuals (89.2% vs. 68.1%)
- SPR is defined as the percent (%) of subjects who achieve anti-HBs titers ≥ 10 mIU/mL
- In all subjects, age 18+ : 91.4% vs. 76.5% (difference: 14.9%)
- Sci-B-Vac elicited higher geometric mean concentration (GMC) of anti-HBs titers in all subjects compared to Engerix-B:
- 6x higher GMC of anti-HBs titers in all adults age 18+ (1148.2 mIU/mL vs. 192.6 mIU/mL)
- 4-8x higher antibody GMCs were elicited in study subjects with Sci-B-Vac compared to Engerix-B regardless of age (65+ : 410.2 mIU/mL vs. 63.7 mIU/mL), obesity (884.0 mIU/mL vs. 110.0 mIU/mL), or diabetic status (222.3 mIU/mL vs. 41.3 mIU/mL)
A copy of the e-poster is available on the “Events/Presentations” page in the “Investors” section of VBI’s website.
About VBI Vaccines Inc.
VBI Vaccines Inc. (Nasdaq: VBIV) is a commercial-stage biopharmaceutical company developing a next generation of vaccines to address unmet needs in infectious disease and immuno-oncology. VBI is advancing the prevention and treatment of hepatitis B, with: (1) the only 3-antigen hepatitis B vaccine, Sci-B-Vac®, which is approved for use and commercially available in Israel and recently completed its Phase 3 program in the U.S., Europe, and Canada; and (2) an immunotherapeutic in development for a functional cure for chronic hepatitis B. VBI’s enveloped virus-like particle (eVLP) platform technology enables development of eVLPs that closely mimic the target virus to elicit a potent immune response. VBI’s lead eVLP programs include a vaccine immunotherapeutic candidate targeting glioblastoma (GBM), a prophylactic cytomegalovirus (CMV) vaccine candidate, and a prophylactic coronavirus vaccine program. VBI is headquartered in Cambridge, MA, with research operations in Ottawa, Canada, and research and manufacturing facilities in Rehovot, Israel.
Cautionary Statement on Forward-looking Information
Certain statements in this press release that are forward-looking and not statements of historical fact are forward-looking statements within the meaning of the safe harbor provisions of the Private Securities Litigation Reform Act of 1995 and are forward-looking information within the meaning of Canadian securities laws (collectively, “forward-looking statements”). The Company cautions that such statements involve risks and uncertainties that may materially affect the Company’s results of operations. Such forward-looking statements are based on the beliefs of management as well as assumptions made by and information currently available to management. Actual results could differ materially from those contemplated by the forward-looking statements as a result of certain factors, including but not limited to, the impact of general economic, industry or political conditions in the United States or internationally; the impact of the ongoing COVID-19 pandemic on our clinical studies, manufacturing, business plan, and the global economy; the ability to establish that potential products are efficacious or safe in preclinical or clinical trials; the ability to establish or maintain collaborations on the development of therapeutic candidates; the ability to obtain appropriate or necessary governmental approvals to market potential products; the ability to obtain future funding for developmental products and working capital and to obtain such funding on commercially reasonable terms; the Company’s ability to manufacture product candidates on a commercial scale or in collaborations with third parties; changes in the size and nature of competitors; the ability to retain key executives and scientists; and the ability to secure and enforce legal rights related to the Company’s products. A discussion of these and other factors, including risks and uncertainties with respect to the Company, is set forth in the Company’s filings with the SEC and the Canadian securities authorities, including its Annual Report on Form 10-K filed with the SEC on March 5, 2020, and filed with the Canadian security authorities at sedar.com on March 5, 2020, as may be supplemented or amended by the Company’s Quarterly Reports on Form 10-Q. Given these risks, uncertainties and factors, you are cautioned not to place undue reliance on such forward-looking statements, which are qualified in their entirety by this cautionary statement. All such forward-looking statements made herein are based on our current expectations and we undertake no duty or obligation to update or revise any forward-looking statements for any reason, except as required by law.
VBI Contact
Nicole Anderson
Director, Corporate Communications & IR
Phone: (617) 830-3031 x124
Email: IR@vbivaccines.com