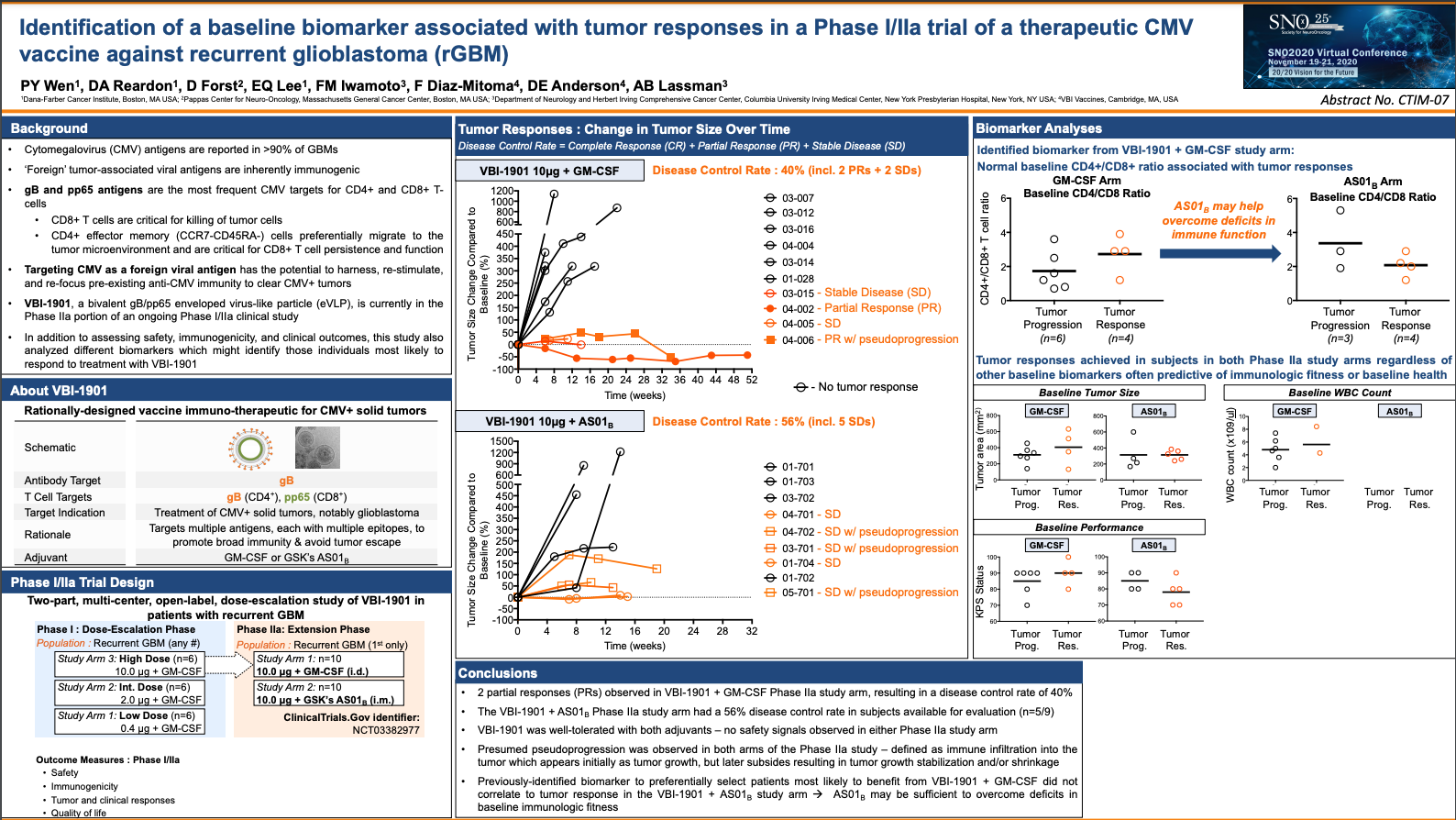
- Interim data show promising disease control rates in patients vaccinated with VBI-1901 combined with the GM-CSF adjuvant (40%), and with VBI-1901 combined with GSK’s adjuvant AS011 (56%)
- 2 partial responses observed in VBI-1901 + GM-CSF study arm, with tumor reduction of more than 50%, and 7 stable disease observations across both vaccinated groups
- VBI-1901 was well-tolerated with both adjuvants – no safety signals observed in either vaccinated group
VBI Vaccines Inc. (Nasdaq: VBIV) (VBI), a commercial-stage biopharmaceutical company developing next-generation infectious disease and immuno-oncology vaccines, today announced Phase 2a (Part B) data from its ongoing Phase 1/2a study of VBI-1901, the company’s cancer vaccine immunotherapeutic candidate designed to target cytomegalovirus (CMV) as a foreign viral antigen in recurrent glioblastoma (GBM). The data were presented in an e-poster at the Society for Neuro-Oncology (SNO) 2020 Annual Meeting, November 19-21, 2020.Data from Phase 2a (Part B) of the ongoing study showed:
- 2 partial responses (PRs) and 2 stable disease (SD) observed in the VBI-1901 + GM-CSF vaccinated group, resulting in a disease control rate of 40% (n=4/10)
- A 56% disease control rate achieved in the group vaccinated with VBI-1901 + AS01, with 5 stable disease observations (n=5/9) – tumor response data for the 10th patient enrolled is pending
- Presumed pseudoprogression was observed in both vaccinated groups– defined as immune infiltration into the tumor which appears initially as tumor growth, but later subsides resulting in tumor growth stabilization and/or shrinkage
Andrew B. Lassman, M.D., Chief of Neuro-oncology at Columbia University Irving Medical Center and Associate Director for Clinical Trials at the Herbert Irving Comprehensive Cancer Center, and principal investigator of the study presented the e-poster, commenting, “Tumor response data is one of the most objective measures of efficacy in open label studies, especially in this difficult-to-treat patient population. Few treatment options are available to recurrent glioblastoma patients, and the tumor response data seen to-date in this ongoing study are encouraging. Any treatment that could demonstrate clinical benefit would be incredibly meaningful.”
Emmanuel Hanon, Senior Vice President, Head of R&D Vaccines at GSK, commented, “The early data seen to-date in this ongoing study are encouraging, underscoring the potential benefit of adjuvants in combination with VBI-1901 in the clinical setting. Previous research in the context of other vaccines has shown AS01’s ability to boost T cell-mediated immunity. This is the first time GSK’s adjuvant system is assessed in oncology and we are looking forward to getting more data about the potential of therapeutic vaccination to treat such an aggressive and recurring disease.”
David E. Anderson, Ph.D., VBI’s Chief Scientific Officer, commented, “This ongoing study continues to demonstrate the potential of VBI-1901, with both the GM-CSF adjuvant and GSK’s AS01 adjuvant system, to be an effective cancer vaccine immunotherapeutic. The tumor responses seen to-date across both study arms, including 2 partial responses and 7 stable disease, are meaningful, especially as an outcome of a monotherapy. This data supports the continued development of the program, both as a monotherapy as well as part of a combination regimen.”
Based on the available data, VBI is exploring a randomized, controlled clinical study, including a potential registration study, for the next phase of development, which could begin in 2021, pending approval from regulatory bodies.
A webcast of Dr. Anderson discussing these data with Jeff Baxter, VBI’s President and CEO, can be found here: https://www.vbivaccines.com/wire/vbi-1901-sno-2020-update/.
A copy of the e-poster is available on the “Events/Presentations” page in the “Investors” section of the VBI Vaccines website.
About the Phase 1/2a Study Design
VBI’s two-part Phase 1/2a study is a multi-center, open-label, dose-escalation study of VBI-1901 in up to 38 patients with recurrent GBM:
- Phase 1 (Part A)
- Dose-escalation phase that defined the safety, tolerability, and optimal dose level of VBI-1901 adjuvanted with granulocyte-macrophage colony-stimulating factor (GM-CSF) in recurrent GBM patients with any number of prior recurrences.
- This phase enrolled 18 recurrent GBM patients across three dose cohorts of VBI-1901: 0.4 µg, 2.0 µg, and 10.0 µg.
- Enrollment completed in December 2018.
- Phase 2a (Part B)
- Subsequent extension of the optimal dose level, 10.0 µg, as defined in the Part A dose escalation phase.
- This phase is a two-arm study, enrolling 10 first-recurrent GBM patients in each vaccinated group, assessing 10.0 µg of VBI-1901 in combination with either GM-CSF or GSK’s proprietary AS01 adjuvant system as immunomodulatory adjuvants.
- Enrollment of the 10 patients in each adjuvant group is complete.
VBI-1901 is administered intradermally when adjuvanted with GM-CSF and intramuscularly when adjuvanted with GSK’s AS01 adjuvant system. Patients in both phases of the study receive the vaccine immunotherapeutic every four weeks until tumor progression.
About VBI-1901 and GBM
VBI-1901 is a novel cancer vaccine immunotherapeutic candidate developed using VBI’s enveloped virus-like particle (eVLP) technology to target two highly immunogenic cytomegalovirus (CMV) antigens, gB and pp65. Scientific literature suggests CMV infection is prevalent in multiple solid tumors, including glioblastoma (GBM). GBM is among the most common and aggressive malignant primary brain tumors in humans. In the U.S. alone, 12,000 new cases are diagnosed each year. The current standard of care for treating GBM is surgical resection, followed by radiation and chemotherapy. Even with aggressive treatment, GBM progresses rapidly and has a high mortality.
About VBI Vaccines Inc.
VBI Vaccines Inc. (Nasdaq: VBIV) is a commercial-stage biopharmaceutical company developing a next generation of vaccines to address unmet needs in infectious disease and immuno-oncology. VBI is advancing the prevention and treatment of hepatitis B, with the only 3-antigen hepatitis B vaccine, Sci-B-Vac®, which is approved for use and commercially available in Israel, and recently completed its Phase 3 program in the U.S., Europe, and Canada, and with an immunotherapeutic in development for a functional cure for chronic hepatitis B. VBI’s enveloped virus-like particle (eVLP) platform technology enables development of eVLPs that closely mimic the target virus to elicit a potent immune response. VBI’s lead eVLP programs include a vaccine immunotherapeutic candidate targeting glioblastoma (GBM), a prophylactic cytomegalovirus (CMV) vaccine candidate, and a prophylactic coronavirus vaccine program. VBI is headquartered in Cambridge, MA, with research operations in Ottawa, Canada, and research and manufacturing facilities in Rehovot, Israel.
Cautionary Statement on Forward-looking Information
Certain statements in this press release that are forward-looking and not statements of historical fact are forward-looking statements within the meaning of the safe harbor provisions of the Private Securities Litigation Reform Act of 1995 and are forward-looking information within the meaning of Canadian securities laws (collectively, “forward-looking statements”). The Company cautions that such statements involve risks and uncertainties that may materially affect the Company’s results of operations. Such forward-looking statements are based on the beliefs of management as well as assumptions made by and information currently available to management. Actual results could differ materially from those contemplated by the forward-looking statements as a result of certain factors, including but not limited to, the impact of general economic, industry or political conditions in the United States or internationally; the impact of the recent COVID-19 outbreak on our clinical studies, manufacturing, business plan, and the global economy; the ability to establish that potential products are efficacious or safe in preclinical or clinical trials; the ability to establish or maintain collaborations on the development of therapeutic candidates; the ability to obtain appropriate or necessary governmental approvals to market potential products; the ability to obtain future funding for developmental products and working capital and to obtain such funding on commercially reasonable terms; the Company’s ability to manufacture product candidates on a commercial scale or in collaborations with third parties; changes in the size and nature of competitors; the ability to retain key executives and scientists; and the ability to secure and enforce legal rights related to the Company’s products. A discussion of these and other factors, including risks and uncertainties with respect to the Company, is set forth in the Company’s filings with the SEC and the Canadian securities authorities, including its Annual Report on Form 10-K filed with the SEC on March 5, 2020, and filed with the Canadian security authorities at sedar.com on March 5, 2020, as may be supplemented or amended by the Company’s Quarterly Reports on Form 10-Q. Given these risks, uncertainties and factors, you are cautioned not to place undue reliance on such forward-looking statements, which are qualified in their entirety by this cautionary statement. All such forward-looking statements made herein are based on our current expectations and we undertake no duty or obligation to update or revise any forward-looking statements for any reason, except as required by law.
1The GSK proprietary AS01 adjuvant system contains QS-21 StimulonTM adjuvant licensed from Agenus Inc. (NASDAQ: AGEN)
VBI Contact
Nicole Anderson
Director, Corporate Communications & IR
Phone: (617) 830-3031 x124
Email: IR@vbivaccines.com