- 33% reduction in tumor size observed in one patient in Part B – reduction sustained through two consecutive MRI scans
- 6-month overall survival (OS) now available for Part A demonstrated benefit for vaccine responders (100%) vs. non-responders (63%)
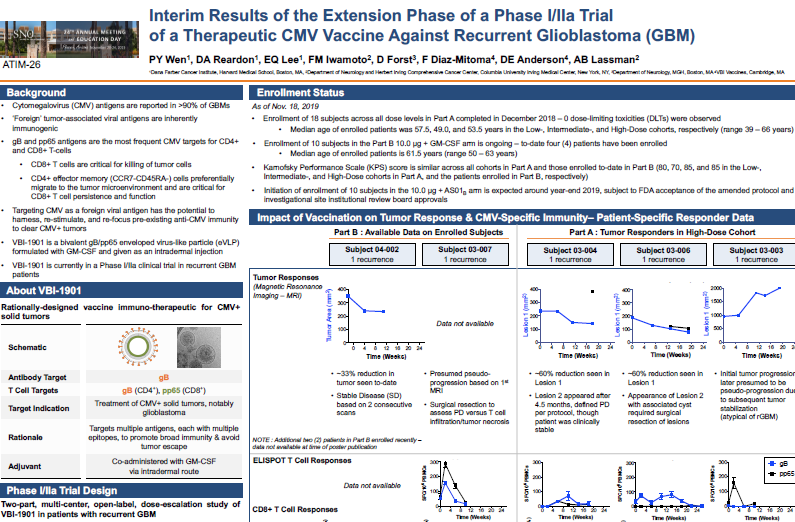
VBI Vaccines Inc. (NASDAQ: VBIV) (VBI), a commercial-stage biopharmaceutical company developing next-generation infectious disease and immuno-oncology vaccines, today announced the presentation of interim data from Part B of the ongoing Phase 1/2a study of VBI-1901, its cancer vaccine immunotherapeutic candidate, in recurrent glioblastoma (GBM) patients in a poster presentation at the 24th Annual Scientific Meeting and Education Day of the Society for Neuro-Oncology (SNO).
Though early in the enrollment process for Part B, the tumor and immunologic responses seen thus far have aligned with the responses and benefit observed in Part A of the study. The second patient enrolled in Part B demonstrates sustained stable disease (SD) after four monthly vaccinations of VBI-1901, following a 33% reduction in tumor size after the first vaccination. Among patients evaluable for response and survival in Part A, follow-up analysis showed the 6-month overall survival (OS) to be 100% in vaccine responders (6/6) compared with 63% in vaccine non-responders (5/8). Median OS in the high-dose cohort of Part A, and more broadly among vaccine responders in Part A, have not yet been reached.
“The clinical outcomes from Part A, the tumor responses we’ve seen in three of the six patients in the high-dose cohort in Part A, and now again the tumor response seen in one of the two patients enrolled and evaluable to-date in Part B continue to be encouraging,” said David E. Anderson, Ph.D., VBI’s Chief Scientific Officer. “In an effort to better understand these early tumor response data, we are also characterizing baseline biomarkers and immunologic responses, notably T cell responses. Correlations between immunological biomarkers and tumor/clinical responses will be refined as more patients are enrolled in Part B of the study.”
To-date, the ongoing Phase 1/2a open-label multicenter study has enrolled all 18 recurrent GBM patients in Part A of the study and four first-recurrent GBM patients in Part B. As of the cut-off date of November 18, 2019, of the four patients enrolled in Part B of the study, two were evaluable for immunologic responses, and one was evaluable for tumor response.
Highlights from Poster Presentation
- Safety
- VBI-1901 continues to be well-tolerated, with no vaccine-related safety signals observed
- Immunogenicity
- The first patient enrolled in Part B demonstrated a robust immune response, including a substantial increase in T cells – though the patient continues to be clinically stable, surgical resection and analysis are ongoing to assess status of tumor response
- The second patient enrolled in Part B demonstrated a 33% reduction in tumor size after the first vaccination of VBI-1901 – classification of stable disease (SD) based on two consecutive Magnetic Resonance Imaging (MRI) scans
- In the high-dose cohort of Part A, vaccine response correlated with tumor response, with all three vaccine responders demonstrating SD for greater than 12 weeks – median overall survival in these patients has not yet been reached
- Two patients in the high-dose cohort of Part A experienced a 60% reduction in the size of primary tumor – VBI-1901 also induced and expanded robust T cell responses in these patients
- Correlations between immunologic biomarkers and tumor/clinical responses will be refined as more patients are enrolled in Part B of the study
The poster presentation is available on the “Events/Presentations” page in the “Investors” section of the VBI Vaccines website.
Poster Presentation/Session Details
- Title: Interim Results of the Extension Phase of a Phase I/IIa Trial of a Therapeutic CMV Vaccine Against Recurrent Glioblastoma (GBM)
- Abstract: ATIM-26
- Date: Friday, November 22, 2019
- Time: 7:30PM – 9:30PM MST
- Location: Marriott Desert Ridge Hotel, Ballroom Lawn
About the Phase 1/2a Study Design
VBI’s two-part Phase 1/2a study is a multi-center, open-label, dose-escalation study of VBI-1901 in up to 38 patients with recurrent GBM:
- Part A
- Dose-escalation phase that defined the safety, tolerability, and optimal dose level of VBI-1901 adjuvanted with granulocyte-macrophage colony-stimulating factor (GM-CSF) in recurrent GBM patients, with any number of prior recurrences.
- This phase enrolled 18 recurrent GBM patients across three dose cohorts of VBI-1901: 0.4 µg, 2.0 µg, and 10.0 µg.
- Enrollment completed in December 2018
- Part B
- Subsequent extension of the optimal dose level, 10.0 µg, as defined in the Part A dose escalation phase.
- This phase is a two-arm study, enrolling 10 first-recurrent GBM patients in each arm, assessing 10.0 µg of VBI-1901 in combination with either GM-CSF or AS01B as immunomodulatory adjuvants.
- Enrollment of the 10 patients in the GM-CSF arm is ongoing, initiation of enrollment of the 10 patients in the AS01B arm is expected around year-end 2019, subject to FDA acceptance of the amended protocol and investigational site institutional review board approvals.
VBI-1901 is administered intradermally when adjuvanted with GM-CSF and will be administered intramuscularly when adjuvanted with AS01B adjuvant system. Patients in both phases of the study will receive the vaccine immunotherapeutic every four weeks until tumor progression.
Additional information, including a detailed description of the study design, eligibility criteria, and investigator sites, is available at ClinicalTrials.gov using identifier NCT03382977.
About VBI Vaccines Inc.
VBI Vaccines Inc. (Nasdaq: VBIV) is a commercial-stage biopharmaceutical company developing a next generation of vaccines to address unmet needs in infectious disease and immuno-oncology. VBI is advancing the prevention and treatment of hepatitis B, with the only commercially-approved trivalent hepatitis B vaccine, Sci-B-Vac®, which is approved for use in Israel and 10 other countries and is currently in a Phase 3 program in the U.S., Europe, and Canada, and with an immunotherapeutic in development for a functional cure for chronic hepatitis B. VBI’s eVLP Platform technology allows for the development of enveloped virus-like particles (eVLP) that closely mimic the target virus to elicit a potent immune response. Integrating its cytomegalovirus (CMV) expertise with the eVLP platform technology, VBI’s lead eVLP program candidates include a prophylactic CMV vaccine candidate and a glioblastoma (GBM) vaccine immunotherapeutic candidate. VBI is headquartered in Cambridge, MA with research operations in Ottawa, Canada and research and manufacturing facilities in Rehovot, Israel.
Cautionary Statement on Forward-looking Information
Certain statements in this press release that are forward-looking and not statements of historical fact are forward-looking statements within the meaning of the safe harbor provisions of the Private Securities Litigation Reform Act of 1995 and are forward-looking information within the meaning of Canadian securities laws (collectively, “forward-looking statements”). The company cautions that such statements involve risks and uncertainties that may materially affect the company’s results of operations. Such forward-looking statements are based on the beliefs of management as well as assumptions made by and information currently available to management. Actual results could differ materially from those contemplated by the forward-looking statements as a result of certain factors, including but not limited to the ability to establish that potential products are efficacious or safe in preclinical or clinical trials; the ability to establish or maintain collaborations on the development of therapeutic candidates; the ability to obtain appropriate or necessary governmental approvals to market potential products; the ability to obtain future funding for developmental products and working capital and to obtain such funding on commercially reasonable terms; the company’s ability to manufacture product candidates on a commercial scale or in collaborations with third parties; changes in the size and nature of competitors; the ability to retain key executives and scientists; and the ability to secure and enforce legal rights related to the company’s products. A discussion of these and other factors, including risks and uncertainties with respect to the company, is set forth in the Company’s filings with the Securities and Exchange Commission and the Canadian securities authorities, including its Annual Report on Form 10-K filed with the Securities and Exchange Commission on February 25, 2019, and filed with the Canadian security authorities at sedar.com on February 25, 2019, as may be supplemented or amended by the Company’s Quarterly Reports on Form 10-Q. Given these risks, uncertainties and factors, you are cautioned not to place undue reliance on such forward-looking statements, which are qualified in their entirety by this cautionary statement. All such forward-looking statements made herein are based on our current expectations and we undertake no duty or obligation to update or revise any forward-looking statements for any reason, except as required by law.
Contacts
Nicole Anderson
Director, Corporate Communications & IR
Phone: (617) 830-3031 x124
Email: info@vbivaccines.com