- PreHevbrio™ [Hepatitis B Vaccine (Recombinant)] launched in the U.S. at the end of Q1 2022 – advancing through commercial stage gates required to enable broad access to the 3-antigen adult hepatitis B (HBV) vaccine
- FDA Orphan Drug Designation received for VBI-1901 for treatment of glioblastoma (GBM) in June 2022
- Two new clinical studies in GBM patients expected to start before the end of 2022
- In partnership with the Government of Canada, new clinical study assessing pan-coronavirus vaccine candidate expected to start in Q3 2022
- Initial Phase 2 human proof of concept combination study data from VBI-2601 + siRNA in chronically infected HBV patients expected by year-end 2022
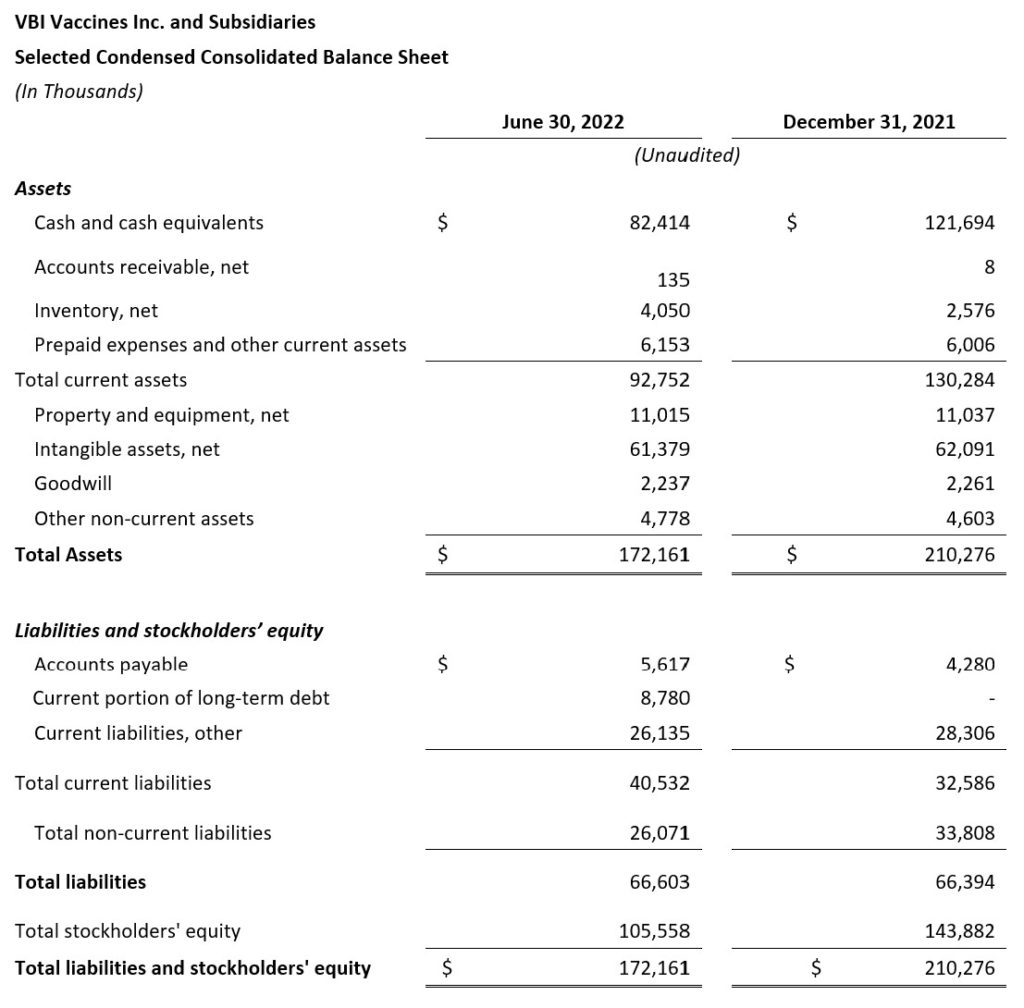
- $82.4 million in cash at the end of Q2 2022
VBI Vaccines Inc. (Nasdaq: VBIV) (VBI), a biopharmaceutical company driven by immunology in the pursuit of powerful prevention and treatment of disease, today announced financial results for the second quarter ending June 30, 2022 and provided a corporate update.
Jeff Baxter, VBI’s President and CEO commented:
“Since the U.S. launch of PreHevbrio, our field teams have been extremely productive, raising awareness of the persistent burden of hepatitis B, the new, recently implemented CDC guidelines for a universal HBV vaccine recommendation for adults aged 19 to 59 years, and of course, the differentiation and value proposition of PreHevbrio. Over recent months:
- We have detailed more than 80% of 3,200 target accounts
- 60% of Medicare-insured lives, 55% of commercially insured lives, and 50% of lives under state Medicaid plans are estimated to have coverage in place for the PreHevbrio specific Current Procedural Terminology (CPT) code
As we advance through these initial commercial stage gates to establish our target network of distribution partners, we believe that as a differentiated 3-antigen HBV vaccine, PreHevbrio will be a meaningful new tool for healthcare providers as they work to tackle hepatitis B.
In addition to the U.S. launch of PreHevbrio, we continue to meet our clinical and regulatory milestones as we advance our lead pipeline candidates against chronic HBV, GBM, and COVID-19, with three new clinical studies expected to start and new therapeutic HBV Phase 2 data expected before the end of 2022. As VBI’s pipeline now includes both commercial and developmental stage assets, we rigorously prioritize our human and financial capital with efficient and well-managed program execution to maximize the impact of our programs in the medical and public health space, and to drive shareholder value.”
Recent Key Program Achievements and Projected Upcoming Milestones
Hepatitis B (HBV)
PreHevbrio™ [Hepatitis B Vaccine (Recombinant)]
- CDC’s Advisory Committee on Immunization Practices’ (ACIP) recommendation of PreHevbrio published in April 2022 Morbidity and Mortality Weekly Report (MMWR), which also included the updated universal HBV vaccination guidelines for adults aged 19-59
- Following marketing authorization in the European Union/European Economic Area and in the United Kingdom, VBI expects to make its 3-antigen HBV vaccine available in select European countries beginning in early 2023 under the name PreHevbri™ [Hepatitis B vaccine (recombinant, adsorbed)]
- Regulatory filing under review by Health Canada
VBI-2601 (BRII-179): HBV Immunotherapeutic Candidate
- Year-end 2022: Interim topline data expected from Phase 2 human proof of concept combination study evaluating safety and efficacy of VBI-2601 (BRII-179) with BRII-835 (VIR-2218), an HBV-targeting siRNA
- H1 2023: Interim topline results expected from two-part Phase 2a/2b combination study evaluating VBI-2601 (BRII-179) as an add-on therapy to standard-of-care treatment
Glioblastoma (GBM)
VBI-1901: Cancer Vaccine Immunotherapeutic Candidate
- June 2022: FDA granted Orphan Drug Designation to VBI-1901 for the treatment of GBM, building on the FDA Fast Track Designation that was granted in June 2021
- Q3 2022: Expected initiation of next phase of development in recurrent GBM setting, aiming to expand the number of patients in the ongoing Phase 1/2a study and adding a control arm, with the potential for accelerated approval based on tumor response rates and improvement in overall survival
- Q4 2022: Evaluation of VBI-1901 in the primary GBM setting expected to initiate as part of the Individualized Screening Trial of Innovative Glioblastoma Therapy (INSIGhT), a Phase 2 adaptive platform trial – data from which have potential to support an accelerated approval application
COVID-19 & Coronaviruses
VBI-2901: Trivalent Pan-Coronavirus Vaccine Candidate
- Q3 2022: Expected initiation of the first clinical study of VBI-2901, which will be supported through Phase 2 clinical development as part of the Company’s partnership with the Strategic Innovation Fund (SIF) of the Canadian Government, through which up to CAD $56 million was earmarked for VBI’s coronavirus vaccine development program
Second Quarter 2022 Financial Results
- Cash Position: VBI ended the second quarter of 2022 with $82.4 million in cash compared with $121.7 million in cash as of December 31, 2021.
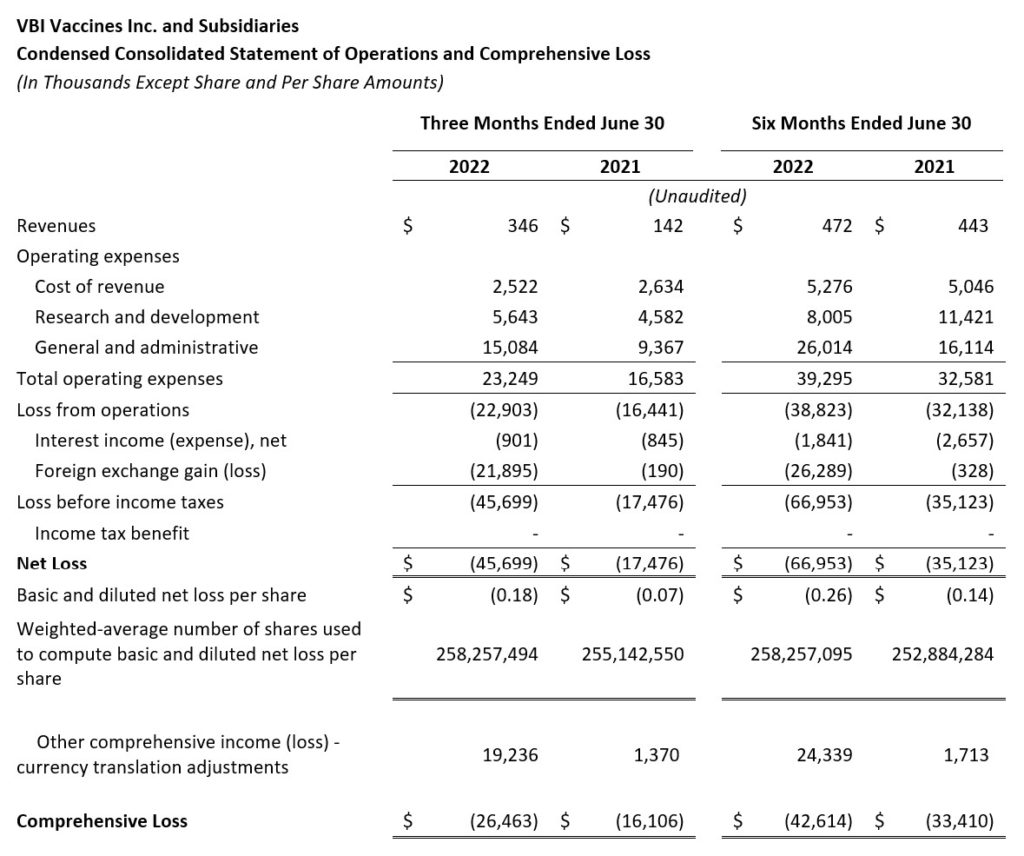
- Revenue: Revenue for the second quarter of 2022 was $0.3 million, compared to $0.1 million for the same period in 2021. The increase was due to the launch of PreHevbrio in the U.S. at the end of the first quarter of 2022, with revenue generation beginning in the second quarter. Over the coming months, VBI expects to expand the number of customers, continuing to broaden access to PreHevbrio in the U.S.
- Cost of Revenue: Cost of revenues was $2.5 million in the second quarter of 2022 as compared to $2.6 million in the second quarter of 2021.
- Research and Development (R&D): R&D expenses for the second quarter of 2022 were $5.6 million compared to $4.6 million for the same period in 2021. R&D expenses were offset by $1.0 million in the second quarter of 2022 and $3.3 million in the second quarter of 2021 by government grants and funding arrangements. The increase in R&D expenses is mainly driven by the advancement of VBI-1901 as we prepare for the next clinical studies in recurrent and primary GBM patients.
- General and Administrative (G&A): G&A expenses for the second quarter of 2022 were $15.1 million compared to $9.4 million for the same period in 2021. The increase in G&A expenses, partially offset by government grants and funding arrangements, was a result of the increased commercial activities related to our 3-Antigen HBV Vaccine, most notably the deployment of our promotional field team and development of our distribution infrastructure. Additional increased costs include increased insurance costs, increased professional costs, and increased labor costs.
- Net Cash Used in Operating Activities: Net cash used in operating activities for the six months ended June 30, 2022, was $37.4 million, compared to $17.4 million for the same period in 2021. The increase was largely due to an increase in net loss attributable to commercial expenses for the launch of PreHevbrio and a decrease in net change in operating working capital as we received $8.3 million of cash in advance from the CEPI Funding Agreement during the six months ended June 30, 2021, compared to $1.0 million cash received in advance from the CEPI Funding Agreement for the same period in 2022.
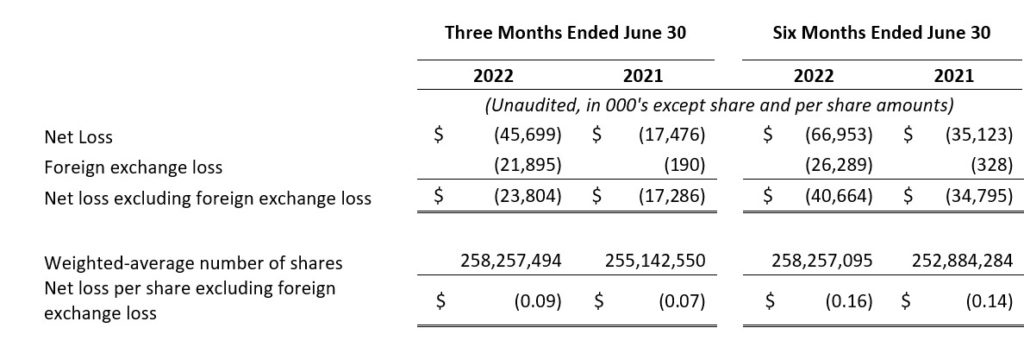
- Net Loss and Net Loss Per Share: Net loss and net loss per share for the second quarter of 2022 were $45.7 million and $0.18, respectively, compared to a net loss of $17.5 million and a net loss per share of $0.07 for the second quarter of 2021.
- Net Loss and Net Loss Per Share, Excluding Foreign Exchange Loss: Net loss and net loss per share, excluding foreign exchange loss, for the second quarter of 2022 were $23.8 million and $0.09, respectively, compared to a net loss and a net loss per share, excluding foreign exchange loss, of $17.3 million and $0.07 for the second quarter of 2021. Foreign exchange loss for the second quarter of 2022 was $21.9 million as compared to $0.2 million for the second quarter of 2021. Certain intercompany loans between VBI Vaccines Inc. and our subsidiaries are denominated in a currency other than the functional currency of each entity. The primary driver of the increase in foreign exchange loss was the impact of the relative strengthening of the U.S. and Canadian Dollars against the New Israeli Shekel upon translation of these intercompany loans.
Use of Non-GAAP Financial Measures
Net Loss Excluding Foreign Exchange Loss and Net Loss per Share Excluding Foreign Exchange Loss are non-GAAP financial measures. VBI’s management believes that the presentation of Net Loss Excluding Foreign Exchange Loss and Net Loss per Share Excluding Foreign Exchange Loss is useful to investors because management does not consider foreign exchange loss, which is primarily driven by changes in exchange rates related to certain intercompany loans, when evaluating VBI’s operating performance. Non-GAAP financial measures are meant to supplement, and to be viewed in conjunction with, GAAP financial results. The presentation of these non-GAAP financial measures should not be considered in isolation or as a substitute for comparable GAAP financial measures and should be read only in conjunction with the Company’s financial statements prepared in accordance with GAAP. Reconciliations of the Company’s non-GAAP measures are included below.
The following represents a reconciliation of Net Loss to Net Loss Excluding Foreign Exchange Loss and Net Loss per Share Excluding Foreign Exchange Loss.
About PreHevbrio™
VBI’s hepatitis B vaccine is the only 3-antigen hepatitis B vaccine, comprised of the three hepatitis B surface antigens of the hepatitis B virus – S, pre-S1, and pre-S2. It is approved for use in the United States, European Union/European Economic Area, United Kingdom, and Israel. The brand names for this vaccine are: PreHevbrio™ (US), PreHevbri™ (EU/EEA, UK), and Sci-B-Vac® (Israel).
Please visit www.PreHevbrio.com for U.S. Important Safety Information for PreHevbrio™ [Hepatitis B Vaccine (Recombinant)], or please see U.S. Full Prescribing Information.
U.S. Indication
PreHevbrio is indicated for prevention of infection caused by all known subtypes of hepatitis B virus. PreHevbrio is approved for use in adults 18 years of age and older.
U.S. Important Safety Information (ISI)
Do not administer PreHevbrio to individuals with a history of severe allergic reaction (e.g. anaphylaxis) after a previous dose of any hepatitis B vaccine or to any component of PreHevbrio.
Appropriate medical treatment and supervision must be available to manage possible anaphylactic reactions following administration of PreHevbrio.
Immunocompromised persons, including those on immunosuppressant therapy, may have a diminished immune response to PreHevbrio.
PreHevbrio may not prevent hepatitis B infection, which has a long incubation period, in individuals who have an unrecognized hepatitis B infection at the time of vaccine administration.
The most common side effects (> 10%) in adults age 18-44, adults age 45-64, and adults age 65+ were pain and tenderness at the injection site, myalgia, fatigue, and headache.
There is a pregnancy exposure registry that monitors pregnancy outcomes in women who received PreHevbrio during pregnancy. Women who receive PreHevbrio during pregnancy are encouraged to contact 1-888-421-8808 (toll-free).
To report SUSPECTED ADVERSE REACTIONS, contact VBI Vaccines at 1-888-421-8808 (toll-free) or VAERS at 1-800-822-7967 or www.vaers.hhs.gov.
Please see Full Prescribing Information.
Condensed Consolidated Financial Statements
About VBI Vaccines Inc.
VBI Vaccines Inc. (“VBI”) is a biopharmaceutical company driven by immunology in the pursuit of powerful prevention and treatment of disease. Through its innovative approach to virus-like particles (“VLPs”), including a proprietary enveloped VLP (“eVLP”) platform technology, VBI develops vaccine candidates that mimic the natural presentation of viruses, designed to elicit the innate power of the human immune system. VBI is committed to targeting and overcoming significant infectious diseases, including hepatitis B, coronaviruses, and cytomegalovirus (CMV), as well as aggressive cancers including glioblastoma (GBM). VBI is headquartered in Cambridge, Massachusetts, with research operations in Ottawa, Canada, and a research and manufacturing site in Rehovot, Israel.
VBI Contact
Nicole Anderson
Director, Corporate Communications & IR
Phone: (617) 830-3031 x124
Email: IR@vbivaccines.com
Cautionary Statement on Forward-looking Information
Certain statements in this press release that are forward-looking and not statements of historical fact are forward-looking statements within the meaning of the safe harbor provisions of the Private Securities Litigation Reform Act of 1995 and are forward-looking information within the meaning of Canadian securities laws (collectively, “forward-looking statements”). The Company cautions that such statements involve risks and uncertainties that may materially affect the Company’s results of operations. Such forward-looking statements are based on the beliefs of management as well as assumptions made by and information currently available to management. Actual results could differ materially from those contemplated by the forward-looking statements as a result of certain factors, including but not limited to, the impact of general economic, industry or political conditions in the United States or internationally; the impact of the ongoing COVID-19 pandemic on our clinical studies, manufacturing, business plan, and the global economy; the ability to successfully manufacture and commercialize PreHevbrio/PreHevbri; the ability to establish that potential products are efficacious or safe in preclinical or clinical trials; the ability to establish or maintain collaborations on the development of pipeline candidates and the commercialization of PreHevbrio/PreHevbri; the ability to obtain appropriate or necessary regulatory approvals to market potential products; the ability to obtain future funding for developmental products and working capital and to obtain such funding on commercially reasonable terms; the Company’s ability to manufacture product candidates on a commercial scale or in collaborations with third parties; changes in the size and nature of competitors; the ability to retain key executives and scientists; and the ability to secure and enforce legal rights related to the Company’s products. A discussion of these and other factors, including risks and uncertainties with respect to the Company, is set forth in the Company’s filings with the SEC and the Canadian securities authorities, including its Annual Report on Form 10-K filed with the SEC on March 7, 2022, and filed with the Canadian security authorities at sedar.com on March 7, 2022, as may be supplemented or amended by the Company’s Quarterly Reports on Form 10-Q. Given these risks, uncertainties and factors, you are cautioned not to place undue reliance on such forward-looking statements, which are qualified in their entirety by this cautionary statement. All such forward-looking statements made herein are based on our current expectations and we undertake no duty or obligation to update or revise any forward-looking statements for any reason, except as required by law.









