- FDA and EMA regulatory review of VBI’s 3-antigen HBV vaccine candidate ongoing – U.S. PDUFA target action date November 30, 2021
- Initiation of the first Phase 1/2 study of VBI-2902, VBI’s monovalent COVID-19 vaccine candidate, expected in Q1 2021, with the anticipated start of a Phase 1/2 study of VBI-2901, VBI’s pan-coronavirus vaccine candidate, expected later in 2021
- Following continued positive Phase 2a data in recurrent GBM patients, expected initiation of randomized study of VBI-1901 in H2 2021, with potential to yield registrational data
- Based on the initial therapeutic HBV data presented, partner Brii Biosciences expects to initiate a combination Phase 2 study of VBI-2601 in Q1 2021
- $119.1 million in cash, cash equivalents, and short-term investments at year-end 2020
VBI Vaccines Inc. (Nasdaq: VBIV) (VBI), a biopharmaceutical company driven by immunology in the pursuit of powerful prevention and treatment of disease, today announced financial results for the fourth quarter and twelve months ended December 31, 2020. The Company also provided a corporate update and its outlook for 2021.
Annual Note from Jeff Baxter, President and CEO:
“2020 was, unfortunately, a historic year, marked by unprecedented disruption, with severe public health, societal, and economic consequence. Every single person, worldwide, felt the devastating effects of the ongoing COVID-19 pandemic. Industries and companies went through extraordinary change and, amidst the temporary adjustments, new normals were established. The impact of this pandemic is likely to be felt for years, if not decades, to come.
The events of 2020 led to impressive collaboration, progress, and transformation across the biotechnology industry, governments, and foundations. We added two new vaccine candidates to our pipeline in 2020 – a multivalent pan-coronavirus vaccine candidate, VBI-2901, and a monovalent COVID-19 vaccine candidate, VBI-2902. To support the advancement of these candidates, we received an award from the Strategic Innovation Fund of the Government of Canada and partnered with both the National Research Council of Canada (NRC), Canada’s largest federal R&D organization, and Resilience Biotechnologies, a Contract Development and Manufacturing Organization. The preclinical results of these two candidates continue to excite us and we are working hard to get these candidates into the clinic in forms that are optimized both for clinical outcome and long-term commercial viability. We recognize the possibility that COVID-19, in some form, may be here to stay, especially with the recent emergence of additional variants, and we are committed to the long-term control of known and emerging coronaviruses.
Our 2020 achievements and progress, however, extend well beyond our coronavirus programs. We successfully completed the pivotal Phase 3 program for our 3-antigen prophylactic hepatitis B (HBV) vaccine candidate and submitted applications for approval in the U.S. and Europe. We believe this vaccine candidate has the potential to be a meaningful intervention for adults in the fight against HBV and we look forward to working with both the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) throughout 2021 as they conduct their review.
In addition to the advancement of these prophylactic vaccine candidates, we continue to see meaningful data generated by the clinical studies of our therapeutic vaccine candidates targeting both chronic HBV infection, VBI-2601, and recurrent glioblastoma (GBM), VBI-1901. With both of these candidates, we are seeking to address diseases that are challenging and aggressive, with few, if any, effective treatment options available to patients. Based on the positive data seen to-date, we and our partners expect to initiate subsequent clinical studies in both indications in 2021.
These achievements are a result of the continued hard work, dedication, and flexibility of every member of the VBI team. Our team remains united across the US, Canada, and Israel in our mission to protect and enhance human life, and we thank our shareholders and partners for their support. With $119.1 million in cash, cash-equivalents, and short-term investments on-hand at the end of 2020, we entered 2021 well-positioned to achieve meaningful milestones across all of our lead pipeline programs over the next 12 months, and beyond.”
Second Half 2020 Key Program Achievements and Projected Upcoming Milestones
3-Antigen Hepatitis B Vaccine Candidate
- November 2020: Biologics License Application (BLA) and Marketing Authorization Application (MAA) submitted to U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA), respectively
- December 2020 and February 2021: EMA acceptance of MAA filing, and FDA acceptance of BLA filing, initiating the review process
- December 2020: Announcement of Syneos Health (Syneos) and VBI partnership for commercialization in the U.S., Europe, and Canada, pending regulatory approvals
- November 30, 2021: U.S. Prescription Drug User Fee Act (PDUFA) target action date set by FDA
VBI-2900: Coronavirus Vaccine Program
- August 2020: Two clinical candidates selected with the goal of bringing forward candidates that add meaningful clinical and medical benefit to those already approved – be it as a one-dose administration, more durable responses, and/or providing broader protection against known and future mutated strains of COVID-19
- VBI-2901 : a trivalent candidate, expressing SARS-CoV-2, SARS-CoV, and MERS-CoV spike proteins
- VBI-2902 : a monovalent candidate, expressing the SARS-CoV-2 spike protein
- September 2020: Through its Strategic Innovation Fund, the Canadian Government agreed to contribute up to CAD$56 million to support VBI-2900 clinical development through Phase 2 studies, to be contributed as expenses are incurred
- December 2020 : Broadened collaboration with NRC to include additional support for pre-clinical evaluation, optimization, and scale-up
- March 2021: Phase 1/2 clinical study of VBI-2902 in adults expected to initiate in Canada
- 2021: Phase 1/2 clinical study of VBI-2901 expected to initiate
VBI-2601 (BRII-179): HBV Immunotherapeutic Candidate
- November 2020: Positive interim Phase 1b/2a proof-of-concept data announced suggesting restoration of antibody and T cell responses
- Q1 2021: Partner, Brii Biosciences, expected to initiate Phase 2 combination study to assess VBI-2601 (BRII-179) and BRII-835 (VIR-2218), a novel RNAi therapeutic, as potential functional cure in chronically infected patients
VBI-1901: Cancer Vaccine Immunotherapeutic Candidate
- November 2020: Positive data announcement from ongoing Phase 1/2a clinical study of VBI-1901 in recurrent GBM patients, including 2 partial responses (PRs), observed with tumor reduction of more than 50%, and 7 stable disease (SD) observations
- H2 2021: Expected initiation of a randomized, controlled clinical study with the potential to yield registrational data
Financing
- Throughout the fourth quarter of 2020, VBI raised total gross proceeds of $15.9 million, issuing 4.8 million shares at an average price of $3.32 through its Open Market Sales AgreementSM, established July 31, 2020 with Jefferies LLC
Financial Results for the Three and Twelve Months Ended December 2020
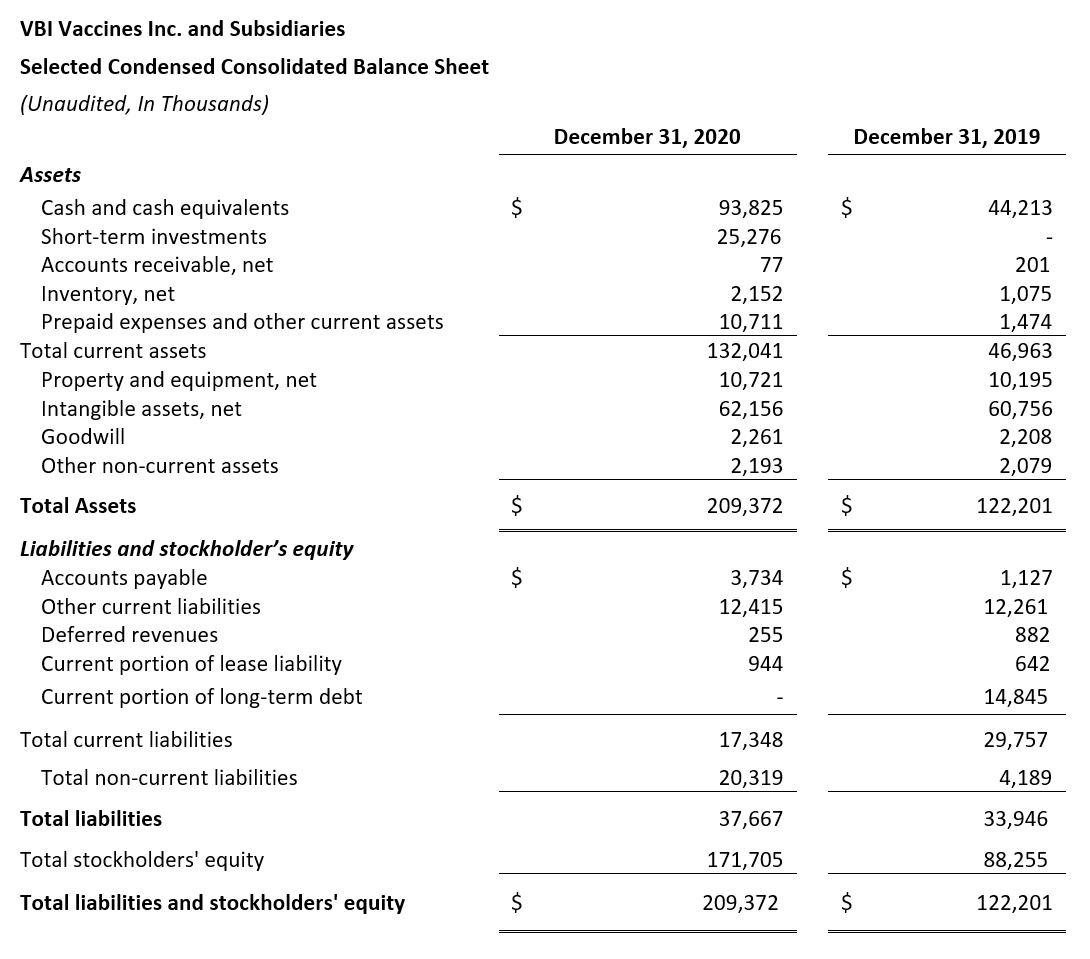
- Cash Position: VBI ended the fourth quarter of 2020 with $119.1 million cash, cash equivalents, and short-term investments compared with $44.2 million as of December 31, 2019.
- Net Cash Used in Operating Activities: Net cash used in operating activities for the full year 2020 was $47.1 million, compared to $48.7 million for the same period in 2019.
- Cash Used for Purchase of Property and Equipment: The purchase of property and equipment in 2020 was $1 million, compared to $3.7 million in 2019. The decrease is a result of the completion of the modernization and capacity increase at the manufacturing facility in Rehovot, Israel.
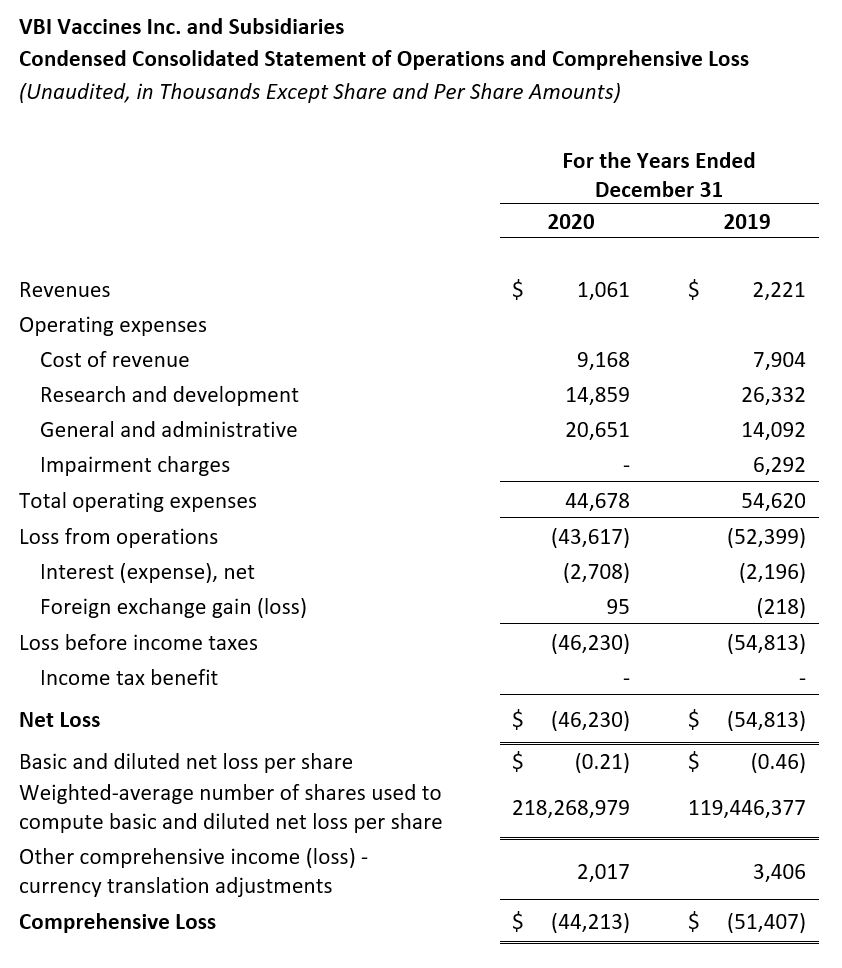
- Revenue: Revenue for the three months ended December 31, 2020 and for the full year 2020 was $0.2 million and $1.1 million, respectively, compared to $0.6 million and $2.2 million for the same time periods in 2019, respectively. There was a decrease in product revenue due to limited product availability as we prepared for our U.S. and Europe regulatory submissions for our 3-antigen HBV vaccine candidate, which occurred in Q4 2020. Additionally, there was a decrease as a result of the license revenue earned as part of the License Agreement with Brii Bio in 2020 compared to 2019.
- Research and Development (R&D): R&D expenses for the fourth quarter and full year 2020 were $4.8 million and $14.9 million, respectively, compared to $4.3 million and $26.3 million for the same periods in 2019, respectively. The decrease in R&D spend in 2020 was primarily due to a decrease in costs related to the Phase 3 clinical studies of our 3-antigen prophylactic HBV vaccine candidate, which were both completed in 2020, but were ongoing in 2019. The decrease in R&D expenses was offset by increased analytical development, manufacturing, and clinical costs associated with our eVLP vaccine candidates.
- General and Administrative (G&A): G&A expenses for the fourth quarter and full year 2020 were $7.1 million and $20.7 million, respectively, compared to $3.8 million and $14.1 million for the same periods in 2019, respectively. The increase in G&A expense in 2020 was primarily due to an increase in pre-commercialization activities related to our 3-antigen prophylactic hepatitis B vaccine, increased insurance costs, and increased people costs.
- Impairment Charge: There were no impairment charges in 2020, compared to a charge of $6.3 million in 2019 related to goodwill.
- Net Loss: Net Loss and net loss per share for the year ended December 31, 2020 were $46.2 million and $0.21, respectively, compared to a net loss of $54.8 million and a net loss per share of $0.46 for the year ended December 31, 2019. The decrease in net loss resulted primarily from decreased R&D expenses offset by the increased cost of revenues and G&A expenses.
About VBI Vaccines Inc.
VBI Vaccines Inc. (“VBI”) is a biopharmaceutical company driven by immunology in the pursuit of powerful prevention and treatment of disease. Through its innovative approach to virus-like particles (“VLPs”), including a proprietary enveloped VLP (“eVLP”) platform technology, VBI develops vaccine candidates that mimic the natural presentation of viruses, designed to elicit the innate power of the human immune system. VBI is committed to targeting and overcoming significant infectious diseases, including hepatitis B, coronaviruses, and cytomegalovirus (CMV), as well as aggressive cancers including glioblastoma (GBM). VBI is headquartered in Cambridge, Massachusetts, with research operations in Ottawa, Canada, and a research and manufacturing site in Rehovot, Israel.
Cautionary Statement on Forward-looking Information
Certain statements in this press release that are forward-looking and not statements of historical fact are forward-looking statements within the meaning of the safe harbor provisions of the Private Securities Litigation Reform Act of 1995 and are forward-looking information within the meaning of Canadian securities laws (collectively, “forward-looking statements”). The Company cautions that such statements involve risks and uncertainties that may materially affect the Company’s results of operations. Such forward-looking statements are based on the beliefs of management as well as assumptions made by and information currently available to management. Actual results could differ materially from those contemplated by the forward-looking statements as a result of certain factors, including but not limited to, the impact of general economic, industry or political conditions in the United States or internationally; the impact of the ongoing COVID-19 pandemic on our clinical studies, manufacturing, business plan, and the global economy; the ability to establish that potential products are efficacious or safe in preclinical or clinical trials; the ability to establish or maintain collaborations on the development of therapeutic candidates; the ability to obtain appropriate or necessary governmental approvals to market potential products; the ability to obtain future funding for developmental products and working capital and to obtain such funding on commercially reasonable terms; the Company’s ability to manufacture product candidates on a commercial scale or in collaborations with third parties; changes in the size and nature of competitors; the ability to retain key executives and scientists; and the ability to secure and enforce legal rights related to the Company’s products. A discussion of these and other factors, including risks and uncertainties with respect to the Company, is set forth in the Company’s filings with the SEC and the Canadian securities authorities, including its Annual Report on Form 10-K filed with the SEC on March 2, 2021, and filed with the Canadian security authorities at sedar.com on March 2, 2021, as may be supplemented or amended by the Company’s Quarterly Reports on Form 10-Q. Given these risks, uncertainties and factors, you are cautioned not to place undue reliance on such forward-looking statements, which are qualified in their entirety by this cautionary statement. All such forward-looking statements made herein are based on our current expectations and we undertake no duty or obligation to update or revise any forward-looking statements for any reason, except as required by law.
VBI Contact
Nicole Anderson
Director, Corporate Communications & IR
Phone: (617) 830-3031 x124
Email: IR@vbivaccines.com









