- PreHevbrio (Hepatitis B Vaccine [Recombinant]) global net revenue increased 52% quarter-over-quarter from Q2 to Q3 2023
- Continued execution across earlier-stage pipeline, including:
- Initiation of Phase 2b study of VBI-1901 in recurrent glioblastoma (GBM) patients
- Interim Phase 1 data announced for pan-coronavirus candidate, VBI-2901
- Novel mRNA-launched eVLP platform technology announced
VBI Vaccines Inc. (Nasdaq: VBIV) (VBI), a biopharmaceutical company driven by immunology in the pursuit of powerful prevention and treatment of disease, today provided a business update and announced financial results for the quarter ended September 30, 2023.
“In Q3, we were focused on pipeline execution with continued revenue growth for PreHevbrio, meaningful data readouts and clinical advancements of our lead development candidates, and the announcement of a next-generation proprietary technology that blends the benefits of our eVLP technology with those of mRNA platforms,” said Jeff Baxter, VBI’s President and CEO. “Additionally, in this period of challenging financial markets for biotechnology companies, we are intensely focused on managing our operating expenses and capital to fuel sustainable growth and value for key stakeholders.”
Recent Key Program Achievements and Projected Upcoming Milestones
Hepatitis B (HBV)
PreHevbrio [Hepatitis B Vaccine (Recombinant)]
- Global net product sales increased 52% from Q2 2023 with $1.1 million earned in Q3 2023
- Net product sales are net of the provision for discounts, chargebacks, rebates, and fees – in the aggregate, these discounts reduced sales by $0.6 million in Q3 2023, from $1.7 million gross sales to $1.1 million net sales
- Quarter-over-quarter momentum continues to grow, with more than a 10% increase in total customer base in Q3 as compared to Q2 2023
- Contracting network across multiple market segments also continues to see growth, including with retail partners, Integrated Delivery Networks (IDNs) and large hospital systems, multiple large military and federal facilities, prisons, and independent and public health clinics
- July 2023: Exclusive licensing deal with Brii Biosciences (Brii Bio) announced for the development and commercialization of PreHevbri in the Asia Pacific region, excluding Japan
- October 2023: Peer-reviewed publication of review of data from studies of PreHevbrio published in Expert Review of Vaccines
- Manuscript title: “PreHevbrio: the first approved 3-antigen hepatitis B vaccine“
- Q4 2023: New market launches expected in additional European Union countries through partnership with Valneva (under brand name PreHevbri®)
VBI-2601 (BRII-179): HBV Immunotherapeutic Candidate
- July 2023: Announced exclusive global licensing agreement with Brii Bio for the development and commercialization of VBI-2601
- This expanded partnership adds VBI-2601 to Brii Bio’s HBV portfolio, which, through a series of strategic investments and partnerships is among the most advanced in the chronic HBV field
- VBI will continue to share in the success of VBI-2601, with the potential to receive regulatory and commercial milestone payments, in addition to potential double-digit royalties on global sales of VBI-2601
- September 2023: Brii Bio announced topline interim results of Phase 2 study evaluating VBI-2601 in combination with pegylated interferon-alpha (PEG-IFNα) in chronic HBV patients
- The combination treatment elicited improved hepatitis B S-antigen (HBsAg) loss and seroconversion vs. PEG-IFNα alone both at the end of treatment and after 12 weeks of follow up
- November 2023: In two late-breaking poster presentations at AASLD The Liver Meeting® 2023, Brii Bio announced new data from Phase 2 studies of VBI-2601 (BRII-179) highlighting progress towards achieving HBV functional cure:
- Direct evidence that BRII-179-induced functional antibody responses can contribute to increased and sustained HBsAg loss rate
- New insight utilizing BRII-179 to enrich patients with intrinsic humoral immune responses for higher HBsAg loss or HBV functional cure rates
Glioblastoma (GBM)
VBI-1901: Cancer Vaccine Immunotherapeutic Candidate
- September 2023: Announcement of first patients dosed in the randomized, controlled Phase 2b study of VBI-1901, an FDA Fast Track and Orphan Drug Designated cancer vaccine candidate, in recurrent GBM patients
- Around Year-End 2023: Expected initiation of VBI-1901 study arm, as part of Individualized Screening Trial of Innovative Glioblastoma Therapy (INSIGhT), a Phase 2 adaptive platform trial, in combination with Agenus Inc.’s anti-PD-1, balstilimab, in primary GBM patients
- H2 2024: Interim data analyses from Phase 2b study in recurrent GBM patients expected, subject to speed of patient enrollment
COVID-19 & Coronaviruses
VBI-2901: Multivalent Pan-Coronavirus Vaccine Candidate
- September 2023: Initial data from Phase 1 study of VBI-2901 reported – the first clinical data from a pan-coronavirus vaccine candidate
- VBI-2901 demonstrated vaccine benefit – reflected as boosting of and/or greater durability of antibody titer maintenance:
- All participants saw boosting and/or high neutralizing antibody responses against a panel of COVID-19 variants and two animal coronaviruses
- Participants with lower baseline antibody titers, reflecting a higher-risk population, saw the greatest boosting effect (5-14x strain-dependent boosting)
- Durability benefit observed regardless of baseline antibody levels, with only about 25% reduction in geometric mean titer (GMT) against the Wuhan strain after 5 months vs. peak responses – compared to an approximate 77% decline in GMT against the Wuhan strain vs. peak responses to a licensed mRNA vaccine [Gilboa M, 2022]1
- VBI-2901 demonstrated vaccine benefit – reflected as boosting of and/or greater durability of antibody titer maintenance:
- Q1 2024: Additional durability and breadth data from the Phase 1 study expected
- Funds from existing partners, including the Canadian government and the Coalition for Epidemic Preparedness Innovations (CEPI) are available to fund the next phase of clinical development
Novel mRNA-Launched eVLP (MLE) Technology Platform
- October 2023: Development of a novel mRNA-launched eVLP (MLE) technology platform announced, supported by preclinical data that have demonstrated significant immunologic and manufacturing benefits
- MLE technology enables the manufacture of particulate vaccines, capable of driving polyfunctional B-cell and T-cell activation, on accelerated timelines, similar to other mRNA vaccine production timelines
- New platform has the potential to be leveraged across infectious disease, cancer, and allergic and autoimmune disease indications
Third Quarter 2023 Financial Results
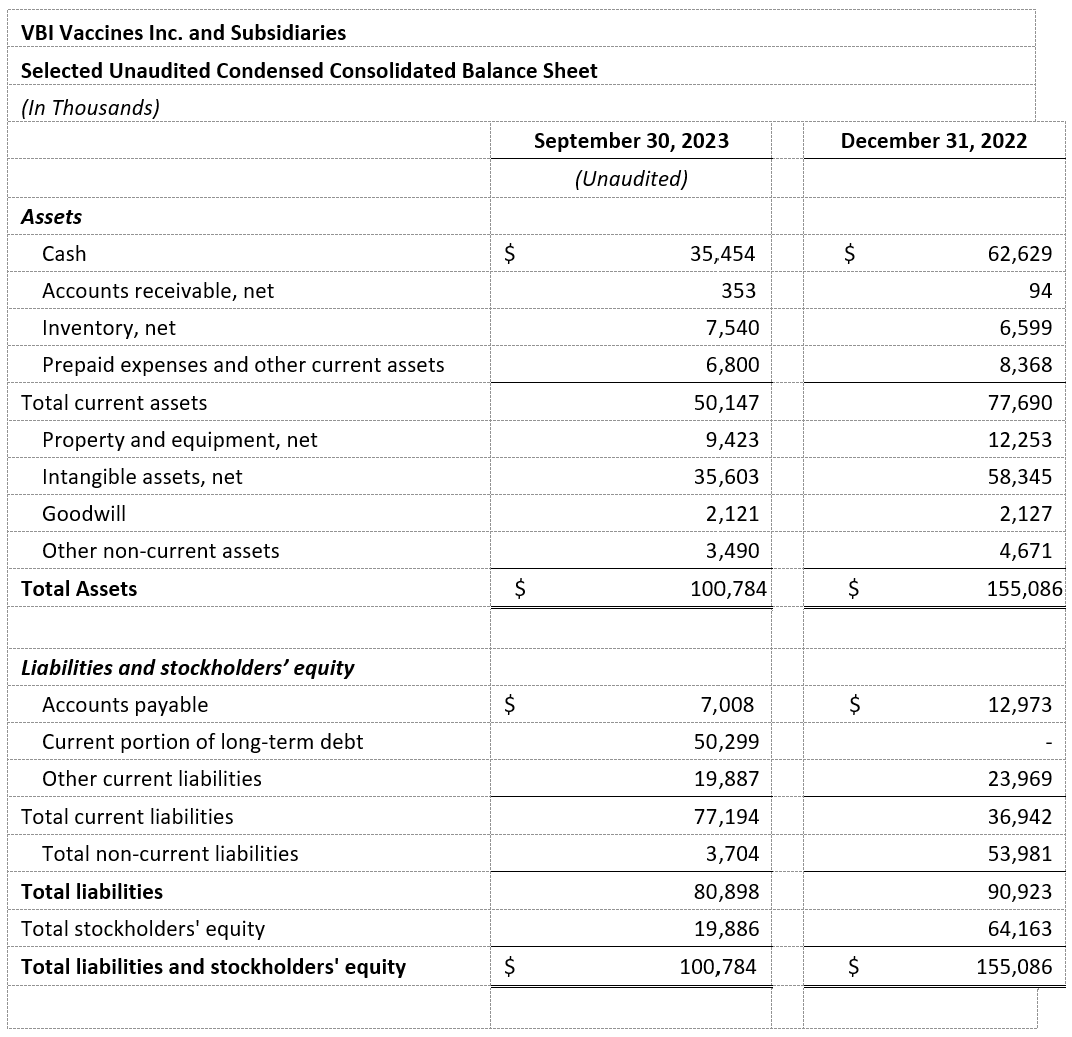
- Cash Position: VBI ended the third quarter of 2023 with $35.5 million in cash as compared with $62.6 million in cash as of December 31, 2022.
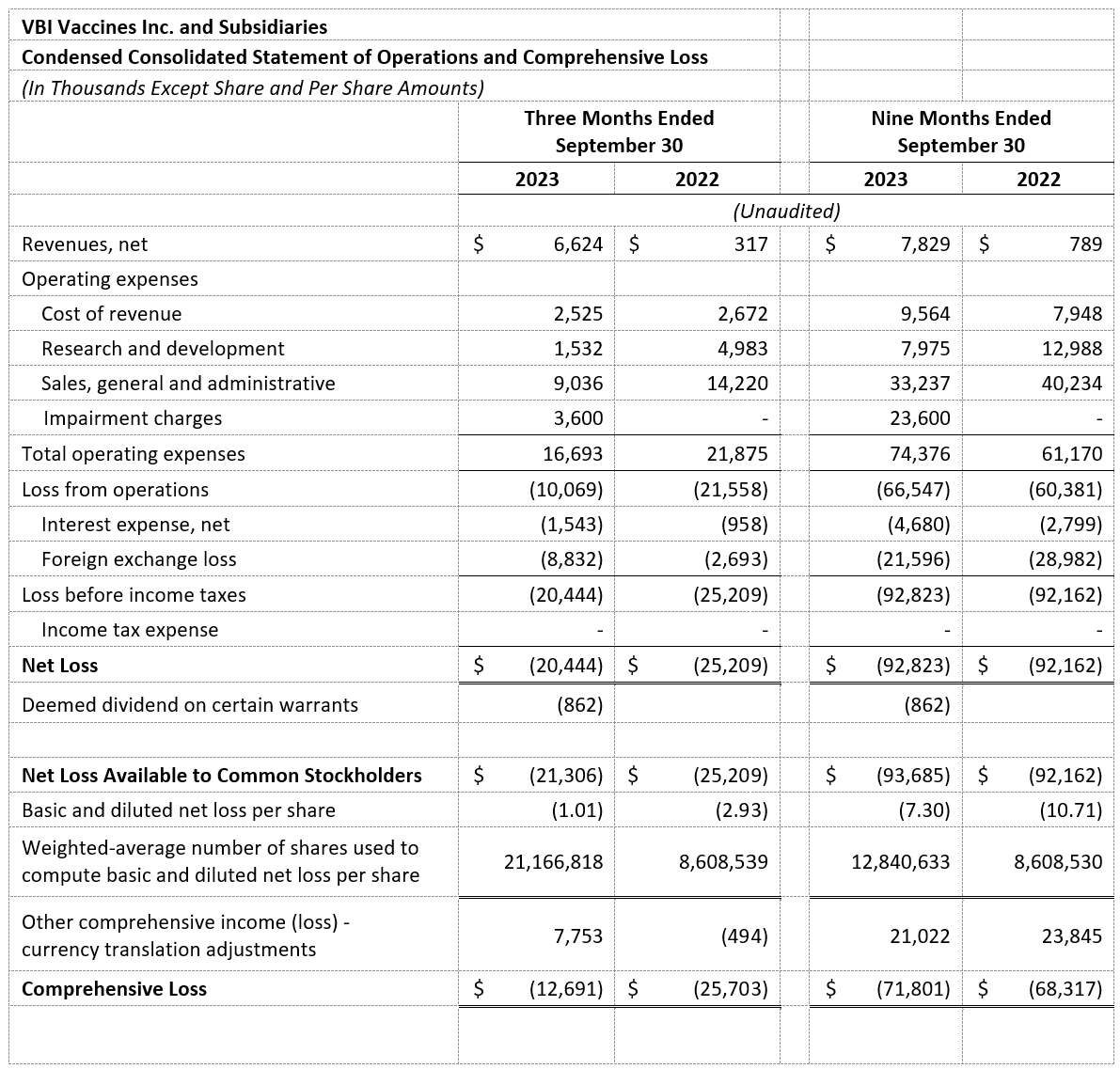
- Revenues, net: Revenues, net for the third quarter of 2023 were $6.6 million as compared to $0.3 million for the same period in 2022. The revenue increase was a result of an increase in product sales of PreHevbrio in the U.S. and of PreHevbri to our partner, Valneva, in Europe, in addition to the license revenue and R&D services revenue associated with the HBV license agreement, expanded in July 2023, with Brii Bio.
- Cost of Revenues: Cost of revenues was $2.5 million in the third quarter of 2023 as compared to $2.7 million in the third quarter of 2022. The decrease in the cost of revenues was due to increased product sales, offset by lower direct labor costs as a result of the recent organization changes that reduced our internal workforce.
- Research and Development (R&D): R&D expenses for the third quarter of 2023 were $1.5 million as compared to $5.0 million for the third quarter of 2022. R&D expenses were offset by $2.7 million in the third quarter of 2023 and $2.4 million in the third quarter of 2022 due to government grants and funding arrangements.
- Sales, General, and Administrative (SG&A): SG&A expenses for the third quarter of 2023 were $9.0 million as compared to $14.2 million for the same period in 2022. The decrease in SG&A expenses was mainly a result of the recent organizational changes that reduced our internal workforce, as announced in April 2023, the redefined deployment strategy of our U.S. commercial field force, and a reduction in activity-based commercial expenses related to PreHevbrio.
- Net Cash Used in Operating Activities: Net cash used in operating activities for the nine months ended September 30, 2023, was $48.8 million compared to $54.6 million for the same period in 2022. The decrease in cash outflows is largely a result of non-cash reconciling items, mainly impairment charges and unrealized foreign exchange loss, and the change in operating working capital, most notably in inventory, other current assets, accounts payable, deferred revenues, and other current liabilities. As announced on April 4, 2023, VBI implemented cost saving measures that are expected to reduce operating expenses from normal business in the second half of 2023 by 30-35% compared to the second half of 2022.
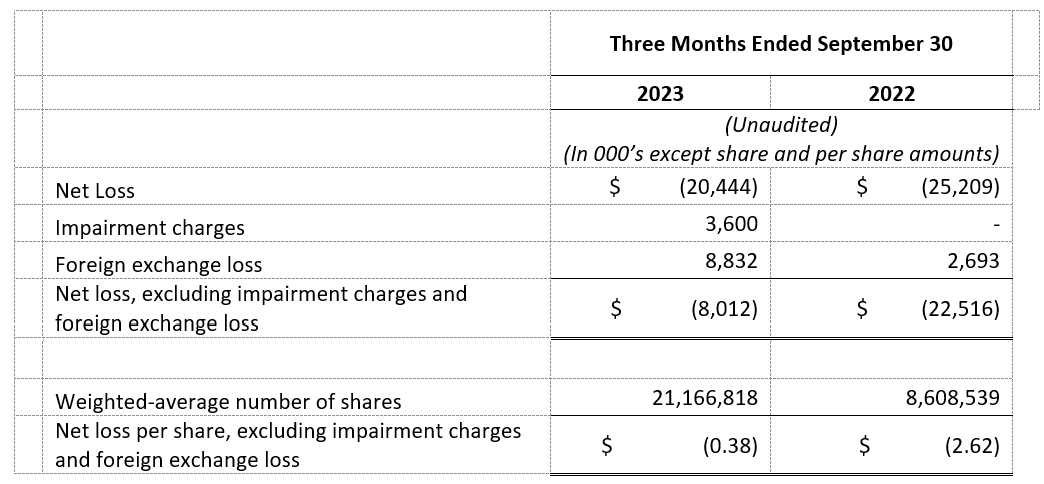
- Net Loss and Net Loss Per Share: Net loss and net loss per share for the third quarter of 2023 were $20.4 million and $1.01, respectively, compared to a net loss and net loss per share of $25.2 million and $2.93 for the third quarter of 2022, respectively.
- Net Loss, Excluding Impairment Charges and Foreign Exchange Loss, and Net Loss Per Share, Excluding Impairment Charges and Foreign Exchange Loss: Net loss, excluding impairment charges and foreign exchange loss, and net loss per share, excluding impairment charges and foreign exchange loss, for the third quarter of 2023 were $8.0 million and $0.38, respectively, compared to $22.5 million and $2.62 for the third quarter of 2022, respectively. See “Non-GAAP Financial Information” below for additional information regarding this non-GAAP financial measure, and “GAAP to Non-GAAP Reconciliation” for a reconciliation of this non-GAAP financial measure to net loss and net loss per share.
- Impairment charges and foreign exchange loss for the third quarter of 2023 were $3.6 million and $8.8 million, respectively, as compared to none and $2.7 million for the third quarter of 2022. Certain intercompany loans between the Company and its subsidiaries are denominated in a currency other than the functional currency of each entity. The primary driver of the increase in foreign exchange loss was the impact of the relative strengthening of the U.S. and Canadian Dollars against the New Israeli Shekel upon translation of these intercompany loans.
Use of Non-GAAP Financial Measures
Net Loss, Excluding Impairment Charges and Foreign Exchange Loss, and Net Loss Per Share, Excluding Impairment Charges and Foreign Exchange Loss, are non-GAAP financial measures and are defined as Net Loss and Net Loss Per Share excluding the non-cash impairment charges and foreign exchange loss in both calculations. Net Loss, Excluding Impairment Charges and Foreign Exchange Loss, and Net Loss Per Share, Excluding Impairment Charges and Foreign Exchange Loss, are not intended to replace Net Loss or Net Loss Per Share or other measures of financial performance reported in accordance with generally accepted accounting principles (GAAP). VBI’s management believes that the presentation of Net Loss, Excluding Impairment Charges and Foreign Exchange Loss, and Net Loss Per Share, Excluding Impairment Charges and Foreign Exchange Loss, are useful to investors because management does not consider foreign exchange loss, which is primarily driven by changes in exchange rates related to certain intercompany loans, and non-cash impairment charges, both of which are non-recurring items, when evaluating VBI’s operating performance. Non-GAAP financial measures are meant to supplement, and to be viewed in conjunction with, GAAP financial results. The presentation of these non-GAAP financial measures should not be considered in isolation or as a substitute for comparable GAAP financial measures and should be read only in conjunction with the Company’s financial statements prepared in accordance with GAAP. Reconciliations of the Company’s non-GAAP measures are included below.
The following represents a reconciliation of Net Loss to Net Loss, Excluding Impairment Charges and Foreign Exchange Loss, and Net Loss Per Share to Net Loss Per Share, Excluding Impairment Charges and Foreign Exchange Loss. See “Non-GAAP Financial Information” below for additional information regarding this non-GAAP financial measure, and “GAAP to Non-GAAP Reconciliation” for a reconciliation of this non-GAAP financial measure to net loss and net loss per share.
About PreHevbrio [Hepatitis B Vaccine (Recombinant)]
PreHevbrio is the only 3-antigen hepatitis B vaccine, comprised of the three surface antigens of the hepatitis B virus – Pre-S1, Pre-S2, and S. It is approved for use in the U.S., European Union/European Economic Area, United Kingdom, Canada, and Israel. The brand names for this vaccine are: PreHevbrio® (US/Canada), PreHevbri® (EU/EEA/UK), and Sci-B-Vac® (Israel).
Please visit www.PreHevbrio.com for U.S. Important Safety Information for PreHevbrio [Hepatitis B Vaccine (Recombinant)], or please see U.S. Full Prescribing Information.
U.S. Indication
PreHevbrio is indicated for prevention of infection caused by all known subtypes of hepatitis B virus. PreHevbrio is approved for use in adults 18 years of age and older.
U.S. Important Safety Information (ISI)
Do not administer PreHevbrio to individuals with a history of severe allergic reaction (e.g. anaphylaxis) after a previous dose of any hepatitis B vaccine or to any component of PreHevbrio.
Appropriate medical treatment and supervision must be available to manage possible anaphylactic reactions following administration of PreHevbrio.
Immunocompromised persons, including those on immunosuppressant therapy, may have a diminished immune response to PreHevbrio.
PreHevbrio may not prevent hepatitis B infection, which has a long incubation period, in individuals who have an unrecognized hepatitis B infection at the time of vaccine administration.
The most common side effects (> 10%) in adults age 18-44, adults age 45-64, and adults age 65+ were pain and tenderness at the injection site, myalgia, fatigue, and headache.
There is a pregnancy exposure registry that monitors pregnancy outcomes in women who received PreHevbrio during pregnancy. Women who receive PreHevbrio during pregnancy are encouraged to contact 1-888-421-8808 (toll-free).
To report SUSPECTED ADVERSE REACTIONS, contact VBI Vaccines at 1-888-421-8808 (toll-free) or VAERS at 1-800-822-7967 or www.vaers.hhs.gov.
Please see Full Prescribing Information.
About VBI Vaccines Inc.
VBI Vaccines Inc. (“VBI”) is a biopharmaceutical company driven by immunology in the pursuit of powerful prevention and treatment of disease. Through its innovative approach to virus-like particles (“VLPs”), including a proprietary enveloped VLP (“eVLP”) platform technology and a proprietary mRNA-launched eVLP (“MLE”) platform technology, VBI develops vaccine candidates that mimic the natural presentation of viruses, designed to elicit the innate power of the human immune system. VBI is committed to targeting and overcoming significant infectious diseases, including hepatitis B, coronaviruses, and cytomegalovirus (CMV), as well as aggressive cancers including glioblastoma (GBM). VBI is headquartered in Cambridge, Massachusetts, with research operations in Ottawa, Canada, and a research and manufacturing site in Rehovot, Israel.
References
1. Gilboa, Mayan, Regev-Yochay, Gili, Mandelboim, Michael et al. Durability of Immune Response After COVID-19 Booster Vaccination and Association With COVID-19 Omicron Infection. JAMA Network Open. September 2022
Cautionary Statement on Forward-looking Information
Certain statements in this press release that are forward-looking and not statements of historical fact are forward-looking statements within the meaning of the safe harbor provisions of the Private Securities Litigation Reform Act of 1995 and are forward-looking information within the meaning of Canadian securities laws (collectively, “forward-looking statements”). The Company cautions that such forward-looking statements involve risks and uncertainties that may materially affect the Company’s results of operations. Such forward-looking statements are based on the beliefs of management as well as assumptions made by and information currently available to management. Actual results could differ materially from those contemplated by the forward-looking statements as a result of certain factors, including but not limited to, the impact of general economic, market, industry or political conditions in the United States or internationally; the impact of the COVID-19 endemic and the continuing effects of the COVID-19 endemic on our clinical studies, manufacturing, business plan, and the global economy; the ability to successfully manufacture and commercialize PreHevbrio/PreHevbri; the ability to establish that potential products are efficacious or safe in preclinical or clinical trials; the ability to establish or maintain collaborations on the development of pipeline candidates and the commercialization of PreHevbrio/PreHevbri; the ability to obtain appropriate or necessary regulatory approvals to market potential products; the ability to obtain future funding for developmental products and working capital and to obtain such funding on commercially reasonable terms; the Company’s ability to manufacture product candidates on a commercial scale or in collaborations with third parties; changes in the size and nature of competitors; the ability to retain key executives and scientists; our ability to regain and maintain compliance with the Nasdaq Capital Market’s listing standards; and the ability to secure and enforce legal rights related to the Company’s products. A discussion of these and other factors, including risks and uncertainties with respect to the Company, is set forth in the Company’s filings with the SEC and the Canadian securities authorities, including its Annual Report on Form 10-K filed with the SEC on March 13, 2023, and filed with the Canadian security authorities at sedar.com on March 13, 2023, as may be supplemented or amended by the Company’s Quarterly Reports on Form 10-Q. Given these risks, uncertainties and factors, you are cautioned not to place undue reliance on such forward-looking statements, which are qualified in their entirety by this cautionary statement. All such forward-looking statements made herein are based on our current expectations and we undertake no duty or obligation to update or revise any forward-looking statements for any reason, except as required by law.
VBI Contact
Nicole Anderson
Director, Corporate Communications & IR
Phone: (617) 830-3031 x124
Email: IR@vbivaccines.com









