- Both co-primary endpoints successfully met – including non-inferiority in all adults age ≥18 years, and superiority in adults age ≥ 45 years
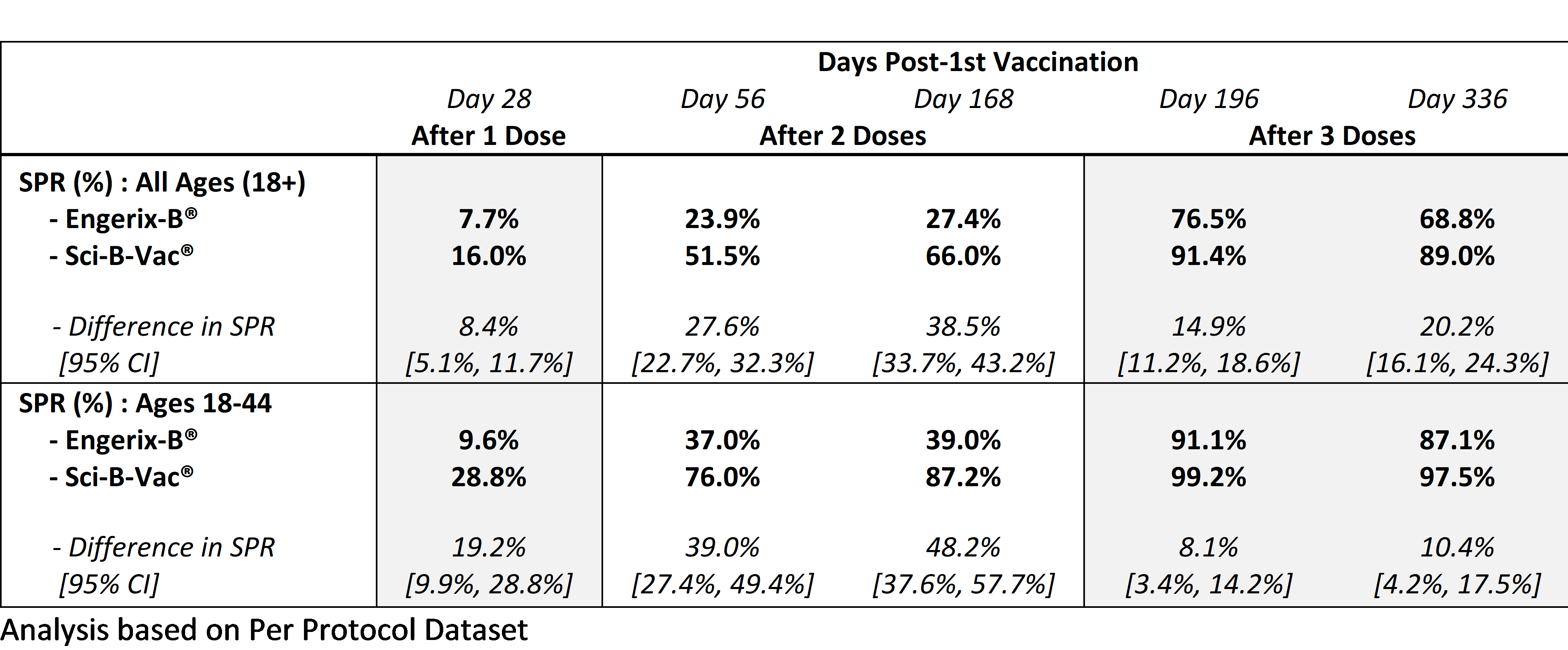
- Seroprotection rates four weeks post-3rd vaccination of Sci-B-Vac® vs. Engerix-B® were statistically significantly higher in key subgroup analyses of adults age ≥ 18 years
- No safety signals observed – safety and tolerability consistent with known profile of the vaccine
- With positive PROTECT data, pending successful completion of CONSTANT study, submissions of BLA/MAA for approval in U.S., Europe, and Canada expected to begin mid-year 2020
- VBI to host conference call and webcast today, Monday, June 17, 2019 at 8:00 AM ET
VBI Vaccines Inc. (Nasdaq: VBIV) (VBI), a commercial-stage biopharmaceutical company developing next-generation infectious disease and immuno-oncology vaccines, today announced positive top-line results from the randomized, double-blind, pivotal Phase 3 study, PROTECT, designed to evaluate the efficacy and safety of a 10 µg dose of Sci-B-Vac®, the company’s trivalent hepatitis B vaccine, compared with a 20 µg dose of the comparator vaccine, Engerix-B®.
The study, which enrolled a total of 1,607 adults, of which approximately 80% were age ≥ 45 years, met both of its co-primary endpoints:
Non-inferiority of seroprotection rate (SPR) of Sci-B-Vac® vs. Engerix-B® in all subjects age ≥ 18 years, 4 weeks after 3rd vaccination (at day 196)
- The SPR in all subjects age ≥ 18 years who received Sci-B-Vac® was 91.4% compared with 76.5% for subjects who received Engerix-B®
- The SPR in the Sci-B-Vac® cohort was statistically significantly higher than the SPR in the Engerix-B® cohort – SPR difference: 14.9%; 95% confidence interval (CI)
- SPR is defined as the percentage of subjects achieving seroprotection, anti-HBsAg levels ≥ 10 mIU/mL
Superiority of SPR of Sci-B-Vac® vs. Engerix-B® in subjects age ≥ 45 years, 4 weeks after 3rd vaccination (at day 196)
- The SPR in subjects age ≥ 45 years who received Sci-B-Vac® was 89.4% compared with 73.1% for subjects who received Engerix-B®
- Superiority of Sci-B-Vac® vs. Engerix-B® was achieved in subjects age ≥ 45 years – SPR difference: 16.4%; 95% CI
Moreover, the SPR of Sci-B-Vac® compared with Engerix-B® was statistically significantly higher in all key subgroup analyses of adults age ≥ 18 years, including by age, gender, body mass index (BMI), diabetic status, and smoking status, 4 weeks after 3rd vaccination (at day 196)
- In diabetics, the SPR in subjects who received Sci-B-Vac® was 83.3% compared with 58.3% for subjects who received Engerix-B® – SPR difference: 25.0%; 95% CI
- In subjects with a body mass index (BMI) > 30, the SPR in subjects who received Sci-B-Vac® was 89.2% compared with 68.1% for subjects who received Engerix-B® – SPR difference: 21.1%; 95% CI
“Hepatitis B is one of the most serious global infectious disease burdens, and successful vaccination of both adults and infants is critical to controlling and, hopefully someday, eradicating the disease,” said Dr. Timo Vesikari, M.D., Ph.D., Director of Vaccine Research Center at the University of Tampere Medical School in Finland, and a principal investigator of the PROTECT and CONSTANT studies. “These results are truly exciting and demonstrate, in a large multicenter controlled trial, the impressive efficacy of Sci-B-Vac® at a dose half that of other hepatitis B vaccines. If approved, this vaccine could play an important role in the prevention of hepatitis B, addressing a significant unmet medical need in the adult population.”
The safety and tolerability seen in PROTECT was consistent with the known safety profile of Sci-B-Vac®, with no new safety risks identified and no safety signals observed in either study cohort. Moreover, there were no observed clusters or unusual patterns of adverse events – the adverse events were generally consistent with characteristics of the study population.
The study did not meet the secondary objective of non-inferiority of two doses of Sci-B-Vac® (at day 168) compared with three doses of Engerix-B® (at day 196) in all subjects age ≥ 18 years, however the SPR of Sci-B-Vac® compared with Engerix-B® was statistically significantly higher at each time point on a per-visit basis:The non-inferiority analysis of two doses of Sci-B-Vac® compared with three doses of Engerix-B® in subjects age 18-45 years will be reassessed based on the complete integrated data analyses from both the PROTECT and the CONSTANT studies. The top-line data from the CONSTANT study is expected around year-end 2019.
Successful completion of the second pivotal Phase 3 study, CONSTANT, is required for the Biologics License Application (BLA) to the U.S. Food and Drug Administration (FDA), the Marketing Authorisation Applications (MAAs) to the European Medicines Agency (EMA), and the New Drug Submission (NDS) to Health Canada.
“We are excited to share the data from this successful head-to-head Phase 3 study, data that are extremely important milestones as we work to increase protection against hepatitis B,” said Francisco Diaz-Mitoma, M.D., Ph.D., VBI’s Chief Medical Officer. “The positive results from PROTECT reaffirm the robust safety and efficacy data that we have for Sci-B-Vac® from previous clinical studies and from its commercial use in Israel, Hong Kong, and several other countries where it is approved or provided on a named-patient basis.”
“On behalf of everyone at VBI, I would like to extend a heartfelt thank you to the study participants and investigators. We are extremely pleased with the top-line efficacy and safety results from PROTECT, which reinforce that Sci-B-Vac® is a potent and safe hepatitis B vaccine with the potential to provide superior protection to a broader adult population,” said Jeff Baxter, VBI’s president and CEO. “We look forward to presenting detailed data in future publications and at medical conferences. We expect top-line results from our second pivotal Phase 3 study, CONSTANT, around year-end 2019 – all subjects have received the final vaccination and safety follow-up visits are underway. Pending successful completion of CONSTANT, we remain on track to submit applications for regulatory approvals in the U.S., Europe, and Canada beginning mid-year 2020.”
 Conference Call and Webcast Details
Conference Call and Webcast Details
VBI Vaccines will host a conference call and webcast with accompanying slides on Monday, June 17, 2019 at 8:00 AM ET. The live webcast and slide presentation can be accessed via the Events/Presentations page in the Investors section of the company’s website, or by clicking this link: https://edge.media-server.com/m6/p/7ryhzgu2.
A replay of the webcast will be archived on the company’s website for 90 days following the live conference call.
To listen to the live conference call, please dial:
- Toll-free U.S. & Canada Dial-In: (866) 602-1050
- International Dial-In: (409) 231-2052
- Conference ID: 7639339
About PROTECT – Safety and Immunogenicity Study
PROTECT is a double-blind, two-arm, randomized, controlled study, that enrolled 1,607 subjects 18 years of age and older. Subjects were randomized in a 1:1 ratio to receive either a three-dose course of Sci-B-Vac® 10 µg or a three-dose course of the control vaccine, Engerix-B® 20 µg. Under the planned dosing schedule, subjects were vaccinated at months zero, one, and six. Enrollment was stratified by age group – age 18-44, age 45-64, and age 65+.
The co-primary endpoints of the PROTECT study were:
- To demonstrate non-inferiority of the SPR induced by Sci-B-Vac® vs. Engerix-B® four weeks after the third vaccination (at day 196) in adults age 18 years and older. Non-inferiority was defined as the lower bound of the 95% CI of the difference between SPR in the Sci-B-Vac® cohort minus the SPR in the Engerix-B® cohort being greater than -5%.
- To demonstrate superiority of the SPR induced by Sci-B-Vac® vs. Engerix-B® four weeks after the third vaccination (at day 196) in adults age 45 and older. Statistical superiority was defined as the lower bound of the same 95% CI being greater than 0%. Clinical superiority was defined as the lower bound of the same 95% CI being greater than 5%.
The study also included multiple secondary endpoints to evaluate the speed to seroprotection, including assessment after two doses of Sci-B-Vac® vs. three doses of Engerix-B®, and the overall safety and tolerability of Sci-B-Vac® vs. Engerix-B®.
About CONSTANT – Lot-to-Lot Consistency Study
CONSTANT is a double-blind, four-arm, randomized, controlled study. Approximately 2,900 adult subjects, age 18-45 years, were randomized in a 1:1:1:1 ratio to receive one of four three-dose cohorts: Lot A of Sci-B-Vac® 10 µg, Lot B of Sci-B-Vac 10 µg, Lot C of Sci-B-Vac 10 µg, or the control vaccine Engerix-B® 20 µg.
The primary objective of this study will be:
- To demonstrate lot-to-lot consistency for immune response, as measured by geometric mean concentration (GMC) of antibodies across three independent, consecutive lots of Sci-B-Vac four weeks after the third vaccination.
The secondary objective will be to evaluate safety and efficacy of Sci-B-Vac® vs. Engerix-B®.
Top-line data from the CONSTANT study is expected around year-end 2019.
About VBI Vaccines Inc.
VBI Vaccines Inc. (Nasdaq: VBIV) is a commercial-stage biopharmaceutical company developing a next generation of vaccines to address unmet needs in infectious disease and immuno-oncology. VBI is advancing the prevention and treatment of hepatitis B, with the only commercially-approved trivalent hepatitis B vaccine, Sci-B-Vac®, which is approved for use in Israel and 10 other countries and is currently in a Phase 3 program in the U.S., Europe, and Canada, and with an immuno-therapeutic in development for a functional cure for chronic hepatitis B. VBI’s eVLP Platform technology allows for the development of enveloped virus-like particle (eVLP) vaccines that closely mimic the target virus to elicit a potent immune response. Integrating its cytomegalovirus (CMV) expertise with the eVLP platform technology, VBI’s lead eVLP program candidates include a prophylactic CMV vaccine candidate and a therapeutic glioblastoma (GBM) vaccine immunotherapeutic candidate. VBI is headquartered in Cambridge, MA with research operations in Ottawa, Canada, and research and manufacturing facilities in Rehovot, Israel.
Cautionary Statement on Forward-looking Information
Certain statements in this press release that are forward-looking and not statements of historical fact are forward-looking statements within the meaning of the safe harbor provisions of the Private Securities Litigation Reform Act of 1995 and are forward-looking information within the meaning of Canadian securities laws (collectively, “forward-looking statements”). The company cautions that such statements involve risks and uncertainties that may materially affect the company’s results of operations. Such forward-looking statements are based on the beliefs of management as well as assumptions made by and information currently available to management. Actual results could differ materially from those contemplated by the forward-looking statements as a result of certain factors, including but not limited to the ability to establish that potential products are efficacious or safe in preclinical or clinical trials; the ability to establish or maintain collaborations on the development of therapeutic candidates; the ability to obtain appropriate or necessary governmental approvals to market potential products; the ability to obtain future funding for developmental products and working capital, and to obtain such funding on commercially reasonable terms; the company’s ability to manufacture product candidates on a commercial scale or in collaborations with third parties; changes in the size and nature of competitors; the ability to retain key executives and scientists; and the ability to secure and enforce legal rights related to the company’s products. A discussion of these and other factors, including risks and uncertainties with respect to the company, is set forth in the Company’s filings with the Securities and Exchange Commission and the Canadian securities authorities, including its Annual Report on Form 10-K filed with the Securities and Exchange Commission on February 25, 2019, and filed with the Canadian security authorities at sedar.com on February 25, 2019, as may be supplemented or amended by the Company’s Quarterly Reports on Form 10-Q. Given these risks, uncertainties and factors, you are cautioned not to place undue reliance on such forward-looking statements, which are qualified in their entirety by this cautionary statement. All such forward-looking statements made herein are based on our current expectations and we undertake no duty or obligation to update or revise any forward-looking statements for any reason, except as required by law.
VBI Contact
Nicole Anderson
Associate, Corporate Communications
Phone: (617) 830-3031 x124
Email: info@vbivaccines.com
VBI Investor Contact
Nell Beattie
Chief Business Officer
Email: IR@vbivaccines.com
VBI Media Contact
Burns McClellan, Inc.
Robert Flamm, Ph.D.
Phone: (212) 213-0006
Email: rflamm@burnsmc.com









